1. INTRODUCTION
About 70 % of Illicium species are distributed in China, especially in Southwestern and Eastern area. Chinese star anise of the species is included in the chinese pharmacopoeia.
Illicium anisatum L. is an evergreen tree belonging to the family Illiciacease and a species of the same genus as the star anise (Illicium verum Hook. f.) that was used as a raw material for the “Tamiflu”, a new influenza treatment.
The species is a toxic plant, distributes throughout eastern Asia where it is used as an ornamental plant, and also found in Jeju Island and southern part of Korean peninsula. The tree contains various kinds of chemical constituents such as sesquiterpene, anisatin and shikimic acid (Yamada et al., 1968).
The bark of Japanese anise is used as a blood coagulant, and the leaves and twigs is used as medicinal herb and fragrance, but the fruit is not used due to toxicty (Yamada et al., 1965).
Recent domestic studies have reported that the tree has the potentials on the aldose reductase inhibition, fat degradation, glycation, anti-elastase activity and anti-inflammation as well as antioxidant activity (Kim and Oh, 1999; Kim and Kim, 2003; Kim and Kang, 2005; Kim et al., 2009; Jeong et al., 2017; Lee et al., 2015; Kim et al., 2015; Jung et al., 2017). However, there is a little studies on the chemical constituents of the tree in domestic because it has toxic although some studies had already done about the biological activities and the analysis of GC-MS on volatile components (Shibuya et al., 1978).
In this study, we investigated the chemical constituents of Japanese anise leaves for future functional use, and elucidated the structures of isolated phenolic compounds.
2. MATERIALS and METHODS
Fresh Japanese anise leaves were collected at Seogwipo, Jeju-do in January 2017, air dried for two weeks and then ground to fine particles to be extracted. The origin of this plant was confirmed by Warm and Subtropical Forest Research Center, National Institute of Forest Service.
The ground leaves (4.37 kg) were immersed in 50 % aqueous acetone at room temperature for 3 days. After three times extraction and filtration, the filtrates were combined together and evaporated on a rotary evaporator under the reduced pressure at 40 °C. The aqueous crude residue was successively fractionated on a separatory funnel and freeze dried after concentration to give n-hexane (0.7107 g), CHCl3 (0.8681 g), EtOAc (3.1115 g), and H2O (16.3765 g) soluble fractions.
1H and 13C NMR spectra, including 2D-NMR such as HSQC (Heteronuclear Single Quantum Coherence) and HMBC (Heteronuclear Multiple Bond Correlation), were recorded on a Bruker (USA) Avance DPX 400 and 700 MHz spectrometers using TMS (tetramethylsilane) as an internal standard and chemical shift was given in δ (ppm).
Thin layer chromatography (TLC) was done on DC-Plastikfolien Cellulose F (Merck) plates and developed with TBAW (t-BuOH-HOAc-H2O (3:1:1, v/v/v)) and 6 % aqueous HOAc. The spot was detected by illuminating ultraviolet light (UV, 254 and 365 nm) and by spraying vanillin reagents (Vanillin-EtOH-H2SO4 (15:250:2.5, w/v/v)), then heating.
A portion of EtOAc (3.12 g) and H2O (6.08 g) soluble fractions were chromatographed on a Sephadex LH-20 column, successively eluting with MeOH-H2O (1:9 → 3:7 → 5:5 → 7:3 → 9:1, v/v) to divide 5 fractions, respectively. (Fig. 1).
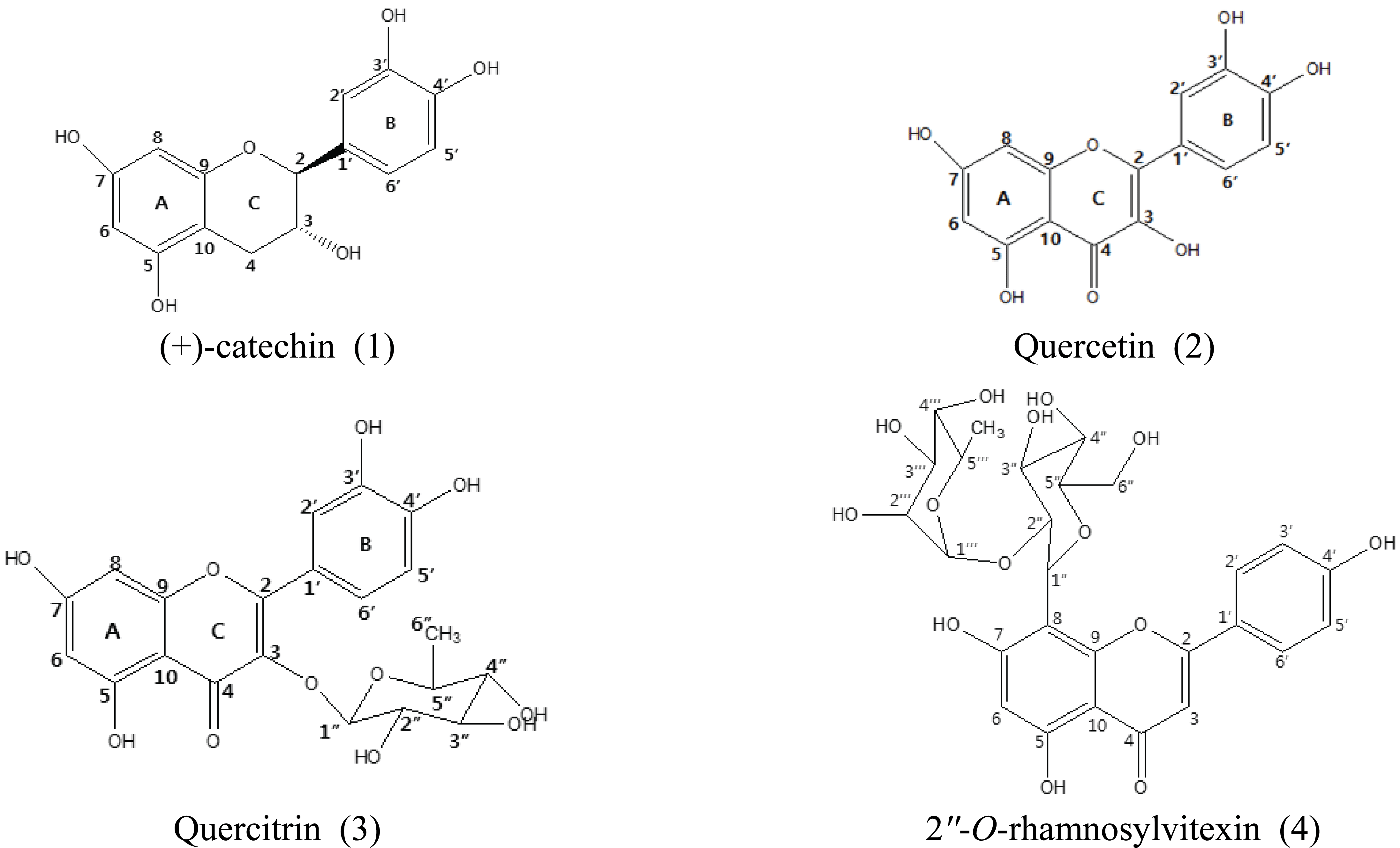
Compound 1, (+)-catechin, (224.5 mg) was isolated by rechrmatography of farction 2 of EtoAc soluble with MeOH-H2O (4:1, v/v). Compound 2, quercetin (79.4 mg), was isolated from fraction 4 of H2O soluble. Fraction 2 of H2O soluble was rechromatographed with MeOH-H2O (4:1, v/v) to isolated the compound 3 (9.2 mg), quercetin-3-O-α-L-(+)-rhamnose which is called quercitrin. Fraction 1 of H2O soluble was retreated with 100 % MeOH, MeOH-H2O (1:1, v/v) and EtOH-hexane (3:1, v/v) to isolated the compound (4) (33.6 mg), apigenin-8-C-rhamnosyl-(1′″→2″)-glucoside which is called 2″-O-rhamnosylvitexin.
The isolated compounds were elucidated as (+)- catechin (1), quercetin (2), quercitrin (3) and 2″-O-rhamnosylvitexin (4) by spectral and literature data, and by comparison with the authentic sample.
Yellowish amorphous powder, Rf : 0.53 (TBAW) and 0.41 (6 % HOAc).
MALDI-TOF-MS : Calculated for C15H14O6 290, Found m/z 313 [M+Na]+, 291 [M+H]+
1H NMR (400 MHz, δ, CD3OD) : 2.51 (1H, dd, J = 8.2 and 16.1 Hz, H-4), 2.85 (1H, dd, J = 5.4 and 16.1 Hz, H-4), 3.98 (1H, m, H-3), 4.57 (1H, d, J = 8.2 Hz, H-2), 5.86 (1H, d, J = 2.3 Hz, H-6), 5.93 (1H, d, J = 1.9 Hz, H-8), 6.75 (1H, dd, J = 1.9 and 8.1 Hz, H-6), 6.76 (1H, d, J = 8.1 Hz, H-5), 6.97 (1H, d, J = 1.9 Hz, H-2).
13C NMR (100 MHz, δ, CD3OD) : 28.55 (C-4), 68.84 (C-3), 82.88 (C-2), 95.53 (C-8), 96.32 (C-6), 100.85 (C-10), 115.28 (C-2′), 116.12 (C-5′), 120.08 (C-6′), 132.24 (C-1′), 146.26 (C-3′), 146.28 (C-4′), 157.86 (C-7), 157.61 (C-5), 156.95 (C-9).
Yellowish amorphous powder, Rf : 0.58 (TBAW) and 0.00 (6 % HOAc).
EI-MS : Calculated for C15H10O7 302, Found m/z 302 [M]+
1H-NMR (400 MHz, δ, CD3OD) : 6.27 (1H, d, J = 2.0 Hz, H-6), 6.53 (1H, d, J = 2.0 Hz, H-8), 7.00 (1H, d, J = 8.5 Hz, H-5′), 7.70 (1H, dd, J = 2.3 and 8.5 Hz, H-6′), 7.82 (1H, d, J = 2.3 Hz, H-2′),
13C-NMR (100 MHz, δ, CD3OD) : 94.45 (C-8), 99.17 (C-6), 104.08 (C-10), 115.71 (C-2′), 116.18 (C-5′), 121.44 (C-6′), 123.71 (C-1′), 136.77 (C-3), 145.93 (C-3′), 147.02 (C-2), 148.43 (C-4′), 157.75 (C-9), 162.28 (C-5), 165.13 (C-7), 176.60 (C-4).
Yellowish amorphous powder, Rf : 0.58 (TBAW) and 0.25 (6 % HOAc).
MALDI-TOF-MS : Calculated for C21H20O11 448, Found m/z 471 [M+Na]+, 449 [M+H]+
1H NMR (400 MHz, δ, CD3OD) : 0.95 (3H, d, J = 6.14 Hz, H-6″), 3.42 (1H, m, H-5″), 3.66 (1H, m, H-4″), 3.76 (1H, dd, J = 3.40 and 3.23 Hz, H-3″), 4.23 (1H, dd, J = 1.63 and 1.66 Hz, H-2″), 5.36 (1H, d, J = 1.5 Hz, H-1″), 6.20 (1H, d, J = 2.0 Hz, H-6), 6.36 (1H, d, J = 2.0 Hz, H-8), 6.91 (1H, d, J = 8.5 Hz, H-5′), 7.31 (1H, dd, J = 2.2 and 8.5 Hz, H-6′), 7.34 (1H, d, J = 2.2 Hz, H-2′).
13C NMR (100 MHz, δ, CD3OD) : 17.69 (C-6″), 71.93 (C-5″), 72.06 (C-3″), 72.13 (C-2″), 73.28 (C-4″), 94.74 (C-8), 99.83 (C-6), 103.56 (C-1″), 105.92 (C-10), 116.39 (C-2′), 116.96 (C-5′), 122.92 (C-6′), 122.99 (C-1′), 136.26 (C-3), 146.42 (C-3′), 149.80 (C-4′), 158.52 (C-9), 159.32 (C-2), 163.21 (C-5), 165.87 (C-7), 179.65 (C-4).
Yellowish amorphous powder, Rf : 0.81 (TBAW) and 0.53 (6 % HOAc).
FAB-MS : Calculated for C27H30O14 578, Found m/z 579 [M+H]+.
1H-NMR (700 MHz, δ, CD3OD) : 0.64 (3H, d, J = 6.3 Hz, H-6′″(CH3)), 2.43 (1H, m, H-5′″), 3.12 (1H, t, H-3′″), 3.39 (1H, dd, J = 2.8 and 9.1 Hz, H-4′″), 3.45 (1H, m, H-5″), 3.63 (2H, m, H-3″,4″), 3.79 (1H, m, H-6″), 3.84 (1H, s, H-2′″), 3.96 (1H, d, J = 11.2 Hz, H-6″), 4.25 (1H, m, H-2″), 5.02 (1H, d, J = 9.9 Hz, H-1″(glc)), 5.09 (1H, d, J = 1.2 Hz, H-1′″(rham)), 6.28 (1H, s, H-6), 6.61 (1H, s, H-3), 6.94 (2H, d, J = 9.1 Hz, H-3’,5’), 7.99 (2H, d, J = 8.4 Hz, H-2’,6’).
13C-NMR (100 MHz, δ, CD3OD) : 16.66 (C-6′″), 61.66 (C-6″), 68.56 (C-5′″), 70.55 (C-4′″), 70.84 (C-2′″), 71.06 (C-4″), 72.14 (C-3′″), 72.32 (C-1″), 76.74 (C-2″), 80.23 (C-3″), 81.53 (C-5″), 98.42 (C-6), 101.14 (C-1′″), 102.25 (C-3), 104.30 (C-10), 104.62 (C-8), 115.60 (C-3′,5′), 122.19 (C-1′), 128.74 (C-2′,6′), 156.55 (C-9), 161.37 (C-5), 141.40 (C-4′), 162.80 (C-7), 165.34 (C-2), 182.79 (C-4).
3. RESULTS and DISCUSSION
The compounds were isolated from the EtOAc soluble and H2O soluble fractions of the extracts of Japanese anise (Illicium ansisatum L) leaves by column chromatography using Sephadex LH-20, and then the structures were elucidated by NMR analysis and by comparison with the authentic literature data.
Compound 1 was obtained as a yellowish amorphous powder from EtOAc soluble fraction.
On the basis of the spectral data, it was identified as (+)-catechin (Foo et al., 1983; Min et al., 2017).
Compound 2 was obtained as a yellowish amorphous powder from EtOAc soluble fraction.
Based on the previous spectral data, the structure was identified as quercetin (3,5,7,3',4'-pentahydroxyflavone) (Kwon et al., 2007; Luo et al., 2009).
Compound 3 was obtained as a yellowish amorphous powder from H2O soluble fraction.
On the basis of the authentic spectral data and by comparison of the literature (pyo et al., 2002; Lee et al., 2004; Min et al., 2017, Lee et al., 2015), it was elucidated as quercetin-3-O-α-L-rhamnopyranose, quercitrin which was first time isolated in this species.
Compound 4 was obtained as a yellowish amorphous powder from H2O soluble fraction.
The 1H NMR spectrum showed that the signals of H-3 and H-6 were singlet at δ 6.61 and δ 6.28, respectively and indicated that there was no adjacent hydrogen. The doublet signals at δ 7.99 were identical to one symmetrical two hydrogen atoms of H-2′ and H-6′ and the coupling constant was 8.4 Hz indicating the ortho-coupled hydrogens in the phenolic B-ring. H-3′ and H-5′ signals of B-ring also showed another symmetrical doublet signals at δ 6.94 with 9.1 Hz of coupling constant.
In the sugar moieties, one signal at δ 5.02 corresponds to H-1″ of glucose bonded to C-8 of A-ring, which was more downfield shifted than the other hydrogen signals of glucose unit, and the coupling constant was 9.9 Hz indicating a β structure The H-1′″ of rhamnose gave one doublet signal at δ 5.09 with 1.2 Hz of J value, indicating α-L-rhamnose. The characteristic methyl signal of rhamnose indicated at δ 0.64. The remaining glucose and rhamnose protons gave complicated typical signals between δ 3.0 and δ 4.26.
In the 13C-NMR spectrum, C-2 and C-3 of heterocyclic C-ring appeared at 165.34 ppm and 102.25 ppm, respectively. C-4 carbonyl carbon gave a peak at 182.79 ppm, indicating a distinctive form of flavone C-ring (Roh et al., 2007). C-6 and C-8 of A-ring appeared at 98.42 and 104.62 ppm, respectively, indicating the typical C-8 of 2″-O-rhamnosylvitexin, about 10 ppm more downshifted than C-8 of apigenin reported by Owen et al. (2003). Therefore, this fact can suggest that glucose C-1″ and C-8 of A-ring may be linked together. C-10 gave a signal at 104.30 ppm and the hydroxyl containing C-5, C-7 and C-9 indicated at 161.37, 162.80 and 156.55 ppm, respectively.
In the B-ring of the aglycon, C-1′ showed a signal at 122.19 ppm and two pairs of symmetrical carbons, C-2′/C-6′ and C-3′/C-5′ gave two strong peaks at 128.74 ppm and 115.60 ppm, respectively. The hydroxyl containing C-4′ was observed at 182.79 ppm and indicated that B ring is symmetrical.
In the sugar moieties, the glucosyl C-1″ was indicated at 72.32 ppm. Also C-2″ shown a signal at 76.74 ppm that was about 8 ppm more downshifted than the C-2″ of vitexin isolated by Lew et al. (1998). The rest of glucosyl carbons appeared at 80.23, 71.06, 81.53 and 61.66 ppm for C-3″, C-4″, C-5″ and C-6″, respectively.
The C-1′″ of rhamnose gave a signal at 101.14 ppm and C-6′″ gave a typical methyl signal at 16.66 ppm. The remaining rhamnosyl carbons resonated at 70.84, 72.14, 70.55 and 68.56 ppm for C-2′″, C-3′″, C-4′″ and C-5′″, respectively.
In the glucosyl HSQC spectrum, H-1″ (δ 5.02) and H-2″ (δ 4.25) were resonated with C-1″ (72.37 ppm) and C-2″ (76.16 ppm). H-3″ and H-4″ (δ 3.63) were correlated with C-4″ (71.06 ppm) and C-3″ (80.23 ppm). Also H-5″ (δ 7.45) and C-5″ (81.53 ppm), H-6″ (δ 3.79 and δ 3.96) and C-6″ (61.66 ppm), had relationship each other, respectively.
In the rhamnose, H-1′″ (δ 5.09) was resonated with C-1′″ (101.14 ppm). H-2′″ (δ 3.84), H-3′″ (δ 3.12), H-4′″ (δ 3.39), H-5′″ (δ 2.43) and H-6′″ (δ 0.64) showed correlations with C-2′″ (70.94 ppm), C-3′″ (72.14 ppm), C-4′″ (70.44 ppm), C-5′″ (68.56 ppm) and C-6′″ (16.66 ppm), respectively.
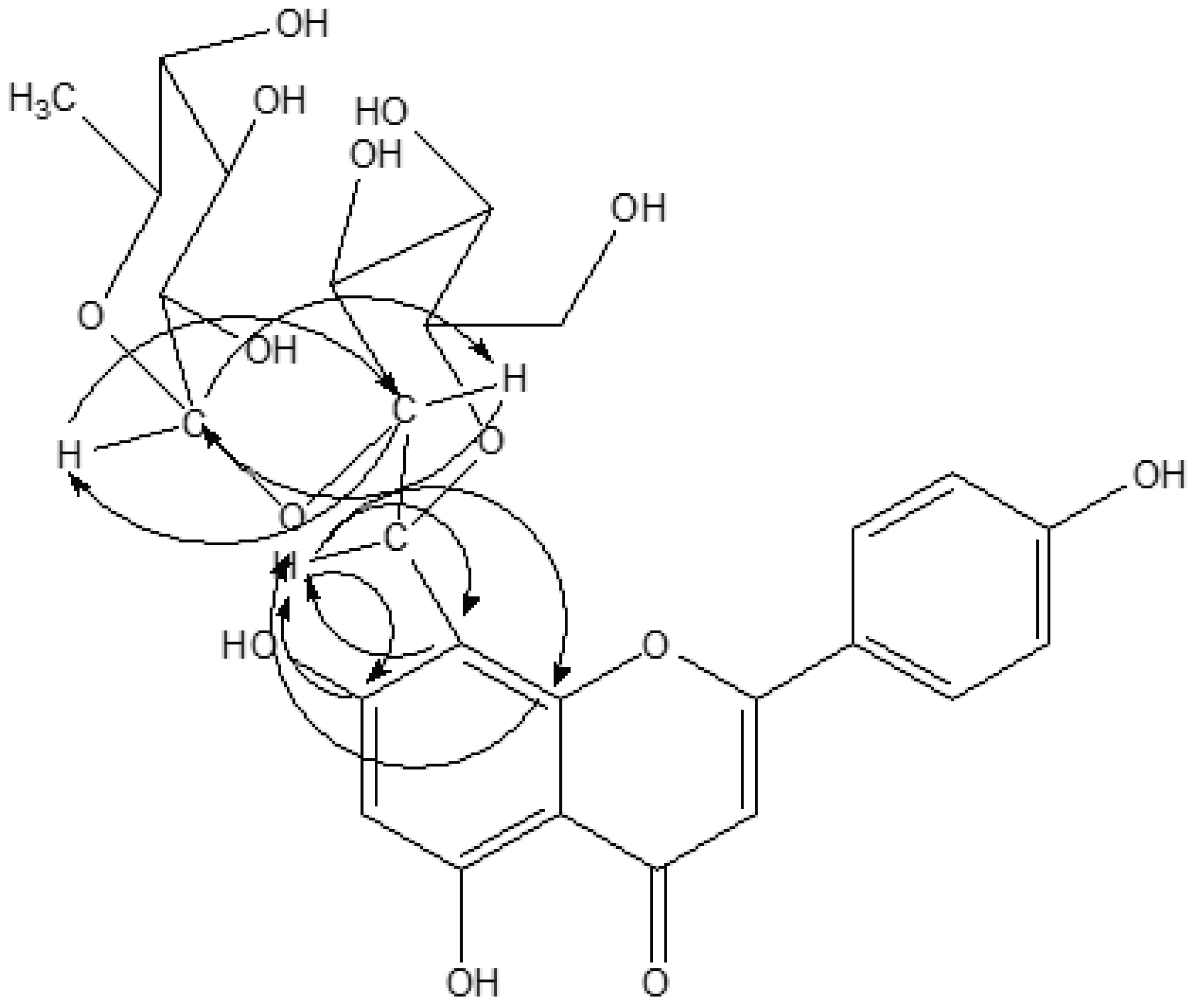
In the glucosyl HMBC spectrum (Fig. 2), H-1″ (δ 5.02) indicated correlations with glucosyl C-2″ (76.74 ppm), apigenin C-7 (162.80 ppm), C-8 (104.62 ppm) and C-9 (151.55 ppm), respectively. H-2″ (δ 25) was also correlated with glucosyl C-1″ (72.32 ppm) and rhamnosyl C-1′″ (101.14 ppm), respectively. C-2″ (76.74 ppm) also showed relationships with H-1″ (δ 5.02) and rhamnosyl H-1′″ (δ 5.09), respectively.
In the rhamnosyl HMBC spectrum, H-1′″ (δ 5.09) indicated the correlation with C-5′″ (68.56 ppm) and glucosyl C-2″ (76.74 ppm). Rhamnosyl C-1′″ (101.14 ppm) also was resonated together with H-2″ (δ 4.25) and H-3′″ (δ 3.84).
Based on the above spectral data, compound 4 was elucidated as 2″-O-rhamnosylvitexin reported by Kwon (2005) and compared with the previous literature data by kim et al. (1997) and kassem et al. (2000).
This compound is isolated, for the first time, from the extractives of Japanese anise (Illicium anisatum L.) leaves.
4. CONCLUSION
Japanese anise (Illicium anisatum L) leaves were collected, air-dried and extracted with 50 % aqueous acetone. The extracts were concentrated and then sequentially fractionated with n-hexane, CHCl3, EtOAc, and H2O to be freeze-dried. A portion of EtOAc soluble (3.12 g) and H2O soluble fractions (6.08 g) were chromatographed on a Sephadex LH-20 column by the successively elution with various aqueous MeOH solution (1:9 → 3:7 → 5:5 → 7:3 → 9:1, v/v).
Compound 1 was isolated by rechromatography of EtOAC fraction 2. Compound 2 also was separated from H2O fraction 4, and compound 3 and compound 4 were isolated from H2O fraction 1 and 2, respectively. The isolated ones were elucidated as (+)-catechin (1), quercetin (2), quercitrin (3) and 2″-O-rhamnosylvitexin (4) by the comparison with the previous spectral and literature data and by the comparison with the authentic sample.
For the first time, quercitrin (3) and 2″-O-rhamnosylvitexin (4) were isolated from the extracts of Japanese anise leaves and might be used valuable index markers for the species.