1. INTRODUCTION
The use of wood barrels and wood chips plays an integral part in the quality and sensory profile of alcoholic beverages. Traditionally, the wood species considered best for making barrels is oak (Quercus genus). However, as a result of the huge demand for oak barrels, some countries have explored alternative wood materials, such as false acacia, cherry, European and American ash, and mulberry, for use in the aging and maturation of alcoholic beverages (Jordão and Cosme, 2022).
In the Philippines, winemakers, distillers, and brewmasters commonly use plastic, glass and stainless containers due to the unavailability and high costs of importing oak barrels.
The World Health Organization reported that spirits account for the majority of alcoholic beverage consumption in the Philippines (72%), followed by beer (27.3%) and wine (0.4%). With the increasing demand for these alcoholic beverages in the Philippines, the Department of Science and Technology – Forest Products Research and Development Institute (DOST-FPRDI) explored, studied, and developed non-oak wooden barrels made from locally available wood species such as Sandoricum koetjape (Burm. f.) Merr., Swietenia macrophylla King, Acacia mangium Willd., and Eucalyptus camaldulensis Dehnh. for wine aging purposes.
Among these, E. camaldulensis Dehnh. or river red gum (RRG) wood was studied further for its potential as alternative to oak for distilled spirit aging. This tree is native to Australia and widely introduced to the Philippines to serve as a plantation or reforestation species because of its invasive and highly adaptive characteristics that could withstand extreme environmental conditions such as drought and high soil salinity (Rojas-Sandoval and Acevedo-Rodríguez, 2019). Its appropriateness for spirit maturation is further supported by its successful use by several Australian distilling companies. For instance, Woodwater Distillery Pty Ltd’s RRG-matured whisky received a bronze medal at the 2023 World Whisky Awards.
To determine the suitability of this local wood as an alternative to oak for maturing spirits, the effect of toasting levels on the macromolecule’s components (cellulose, hemicellulose, lignin) and extractives of the RRG wood was initially investigated using ground wood samples and model spirit solution through various chemical methods and instrumental analysis. These chemical components are known to degrade as the temperature rises (Kim et al., 2018; Park et al., 2020) and hydrolyze at higher toasting temperature (> 200°C) to form into new aromatic and sensory compounds that could significantly improve the quality, complexity, and sensory profile of alcoholic beverages upon aging (Carpena et al., 2020).
This study will serve as a baseline knowledge for the suitability of different toasting level in E. camaldulensis for the wood industry, specifically for use as wooden barrels and wood chips for the aging of alcoholic beverages.
2. MATERIALS and METHODS
Kiln dried RRG wood samples were prepared following the TAPPI T 257 method. Samples were cut to matchstick size, air-dried, and then ground using a Wiley mill (Wiley Mill Model N02, Arthur Thomas, Chadds Ford, PA, USA). The ground samples were then sieved in a mesh wire passing 40 mesh and retained at 60 mesh. The samples retained on the 60-mesh screen were used to determine chemical properties. Ground samples were then toasted in a convection oven at varying temperatures and durations (Fig. 1; Table 1).
| Toasting level | Temperature and duration |
|---|---|
| Untoasted | No heat treatment |
| Heavy toasting | 200°C, 25 min |
| Charred | 250°C, 15 min |
Then, the samples were subjected to proximate chemical analysis. For other chemical analyses such as total phenolic content (TPC), antioxidant, total sugar analysis and gas chromatography-mass spectrometry (GC-MS) analysis, 20 g/L RRG wood chips (untoasted, heavy, charred) were soaked in 40% ethanol for 1 month (Fig. 2) as an experimental spirit solution.
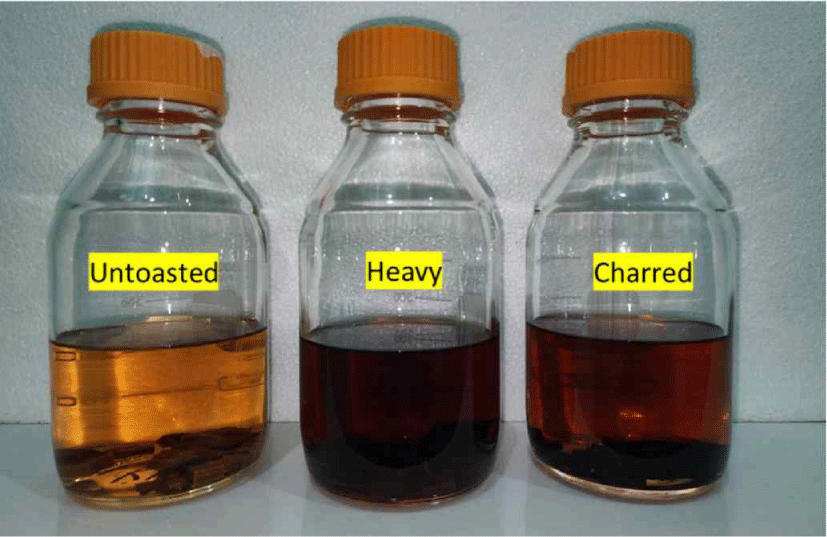
Standard methods (with some modifications) were used for chemical analysis of wood components. All analyses were done in triplicate (Table 2).
| Proximate chemical analysis | Test methods/Reference |
|---|---|
| Moisture content | TAPPI T 264 cm-07 (TAPPI, 2007) |
| Ash content | TAPPI T 211 om-12 (TAPPI, 2012) |
| Ethanol (40%) extractive content | Modified TAPPI T204 cm-07 (TAPPI, 2007; Balagot et al., 2024) |
| Hot water solubility | T 207 cm-08 (TAPPI, 2008) |
| 1% Sodium hydroxide (NaOH) solubility | TAPPI T 212 om-12 (TAPPI, 2012) |
| Lignin content | Modified TAPPI T 222 om-15 (TAPPI, 2011; Balagot et al., 2024) |
| Holocellulose content | Erickson, modified TAPPI procedure (Erickson, 1962; Balagot et al., 2024) |
| Alpha-cellulose content | ASTM-D1103-60 (ASTM, 1968) |
| Hemicellulose content | Difference between holocellulose and alpha-cellulose content |
TPC was determined with the Folin-Ciocalteu reagent according to a procedure described by Singleton and Rossi (1965). Sample (0.5 mL) was added with 0.2 mol/L Folin-Ciocalteu reagent (2.5 mL) followed by 75 g/L sodium carbonate solution (2 mL). The absorbance readings were taken at 765 nm after incubation at 45°C for 15 minutes using UV Vis spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan). Gallic acid was used as a reference standard, and the results were expressed as milligram gallic acid equivalent (mg GAE/g) dried extract. The calibration curve ranged from 0.0–1,000 ug/mL. The data were presented as mean values ± SD (n = 3).
This test using 2,2-diphenyl-1-picrylhydrazyl (DPPH) was carried out following the method described by Sharma et al. (2018) with some modifications. The working solution was prepared using a methanolic solution (98 mg/L). A portion (950 μL) of the working solution was added to the concentrated extracts and standard solutions. The sample was incubated for 30 minutes and absorbance was read at 515 nm using UV Vis spectrophotometer (UV-1700, Shimadzu). Trolox solutions were used for calibration, and antioxidant activity was expressed in mmol TE/g dried extract. The calibration curve ranged from 0.050–0.500 mM.
The method used was from Apak et al. (2008) with some modifications. The analysis, three solutions were mixed in a test tube. Solution A was prepared by dissolving copper (II) chloride in distilled water to produce a solution containing 0.010 M Cu(II). Solution B contained ammonium acetate buffer pH 7.0, which was prepared by dissolving ammonium acetate in distilled water. Solution C contained 0.0075 M neocuproine (2,9-dimethyl-1,10-phenanthroline) in ethanol.
The reaction mixture was left for 30 minutes in the dark and then the absorption was measured at 450 nm using a UV Vis spectrophotometer (UV-1700, Shimadzu). Trolox was used as a reference standard. The results were expressed as Trolox equivalent antioxidant capacity (TEAC) or in mmol TEAC/g dried extract. The calibration curve ranged from 0.0–0.500 mmol/L.
Total sugar content was quantified using the procedure published by DuBois et al. (1956). A 0.5-mL of extracts was put into a test tube and added with an equal amount of 5% phenol. The working solution was vortexed to ensure a complete reaction. Then, 2.5 mL of concentrated sulfuric acid was added and thoroughly vortexed. The solution was allowed to stand for 10 min before cooling in a water bath (25°C–30°C) for 20 min. The absorbance of the sample or standard was measured at 490 nm using a UV Vis spectrophotometer (UV-1700, Shimadzu). A calibration curve with a coefficient determination of 0.9974 was prepared using glucose solutions (0–0.10 mg/mL).
The infrared spectra of untoasted, heavy and charred RRG wood samples were obtained using Fourier transform infrared (FTIR) spectrophotometer equipped with Single Reflection Diamond Attenuated Total Reflectance (ATR; IR Prestige 21, Shimadzu, Japan) in the range of 400–4,000 cm–1 and a resolution of 4 cm–1 with a total of 40 scans per sample (n = 5). The average spectra of each RRG wood sample were plotted using the Origin Pro 2025.
One mL of methanol was transferred into a 2 mL microcentrifuge. This was dried using a centrifugal vacuum concentrator along with the sample. After drying, the blank was reconstituted with 100 μL methanol, then dried with anhydrous sodium sulfate. This was then injected into the GC-MS for analysis.
1 mL of the sample was transferred into a 2 mL microcentrifuge. This was dried using a centrifugal vacuum concentrator along with the blank. After drying, the sample was reconstituted with 100 μL methanol, then dried with anhydrous sodium sulfate. This was then injected into the GC-MS (8890 GC system; 5977B Mass Selective Detector, Agilent, Santa Clara, CA, USA) for analysis (Table 3).
| Oven temperature program: Rate (°C/min) | Temp (°C) | Hold time (min) |
|---|---|---|
| 50 | 0 | |
| 15 | 120 | 0 |
| 10 | 300 | 15 |
3. RESULTS and DISCUSSION
In this study, the effects of toasting on the chemical composition of RRG wood were determined. As presented in Fig. 3, heavy toasted and charred RRG wood exhibit moisture contents of 3.08% and 1.21%, respectively, in contrast to the 8.96% moisture level seen in untoasted RRG wood. This result showed an inverse relationship between the moisture content of RRG wood samples and the application of heat, indicating that moisture content diminishes as temperature rises. The weight loss during the toasting process is expected due to the evaporation of both free and bound water from the wood components (Chatonnet and Escobessa, 2007). Aside from water, other volatile components evaporate from wood when the toasting temperature rises, explaining the weight loss from heavy and charred RRG wood (Kainuma et al., 2024).
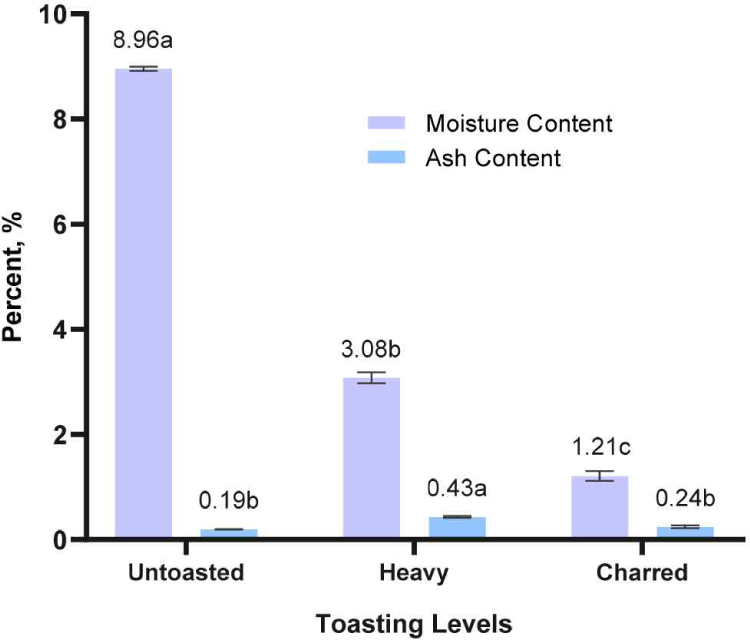
Moisture content of wood has a fundamental role in the oxygen diffusion coefficient of a barrel, which plays an oenological impact on the alcohol being aged (del Alamo-Sanza and Nevares, 2014; Junqua et al., 2021; Sorz and Hietz, 2006; Vivas et al., 2003). However, in this study, wood chips were toasted and not the barrel itself. Future studies will be done on the effect of wood barrel moisture content on the model wine and spirit solution.
The ash contents of the heavily toasted RRG wood sample exhibited the highest value among the varying toasting of wood samples. The closeness of ash content values (0.19%–0.43%) among the untoasted, heavy toasted, and charred RRG wood revealed that toasting treatment cannot degrade the inorganic components of the wood, which include minerals and heavy metals. These components are generally found in trees, having been absorbed from the soil via the roots and then distributed throughout different portions of the plant (Smailagić et al., 2021).
Given that wooden barrels and wood chips are in contact with the alcoholic beverages, the migration of these inorganic materials during maturity will be expected. These minerals and heavy metals are known to influence the maturation of spirits. It could play a crucial catalytic role in oxidation reactions involving phenolic compounds and other substrates in wine spirits. However, its presence on alcoholic beverages raises toxicological or physiological concerns (Sofia et al., 2022). Future studies will determine the composition and contents of the RRG wood ash to make sure that spirit aged with RRG wood are safe for human health.
As presented in Fig. 4, 1% NaOH solution extracted the highest amount of compounds, ranging from 13.20 ± 0.26% to 21.61 ± 0.35%, compared to the other two solvents: hot water (3.57 ± 0.29%–6.83 ± 1.61%) and 40% ethanol solution (5.18 ± 0.57%–7.41 ± 0.48%). This is due to the alkali hydrolyses of ester bond between polysaccharides and lignin which enhances the solubility and release of polysaccharides (Nor Nadiha and Jamilah, 2020). Furthermore, the toasting of RRG wood have a significant effect on the quantity of extracted compounds due to the degradation of these polysaccharides, particularly hemicellulose, that are readily extractable to alkali solution compared to hot water and organic solvents. These wood polysaccharides begin to degrade through heat treatment at around 180°C, with significant changes occurring around 250°C (del Álamo et al., 2008; Esteves and Pereira, 2009) making it more soluble and extracted as new compounds (Kainuma et al., 2024) which explains the significant increase of extracted compounds in charred RRG using 1% NaOH solution.
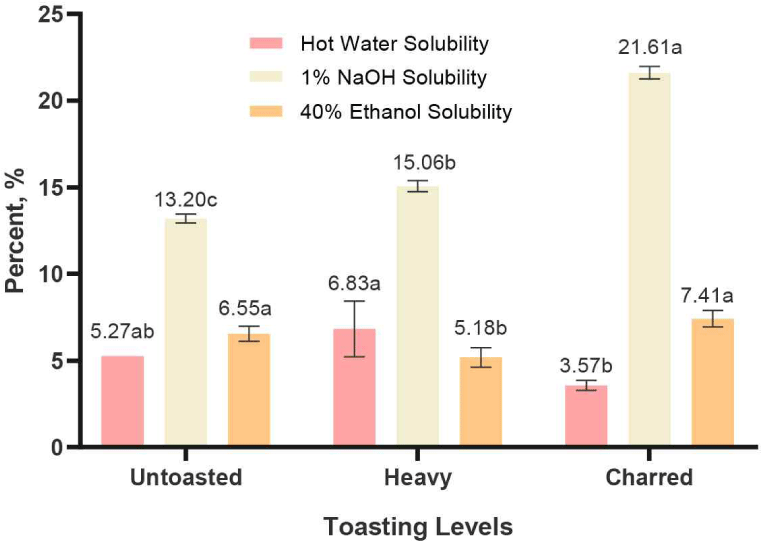
Hot water extracted tannins, sugars, dyes, and starches from the wood. The partial hydrolysis of hemicellulose occurs during the hot water extraction process, which results in the solubility of starch in hot water, and contributes to the hot water solubility content (Iswanto et al., 2021). However, as presented in Fig. 4, heavy toasting of RRG wood resulted in the increase of the extracted hot water-soluble compounds (6.83 ± 1.61%), which can be due to the partial breakdown of some hemicellulose and lignin components into new, smaller and more soluble compounds (Gašparovič et al., 2010; Petrozziello et al., 2020). The charred RRG had the lower extractable compounds in hot water solution which can be due to the thermal denaturation of these soluble sugars and transformed into volatile compounds (Le Floch et al., 2015) which possibly volatilized during the high toasting and high temperature extraction process.
A 40% ethanol solution was also used in the study as a model for a spirit beverage, as it can extract compounds similar to those found in alcoholic spirits during aging. As presented in Fig. 4, there was a significant difference between heavy toasted (5.18 ± 0.57%) and charred (7.41 ± 0.48%) RRG wood. This could be due to the extraction of water-soluble hemicellulose and lignin derivative compounds from the high toasting process. Future studies will measure these non-volatile compounds using high performance liquid chromatography (HPLC) to further determine the effect of toasting on individual polyphenols and other non-volatile compounds.
It is known that major wood components such as lignin and cellulose do not directly affect the sensorial characteristics of the aged alcoholic beverages. However, pyrolysis or toasting of these wood components (Figs. 5 and 6) during wooden barrel and wood chips production, may lead to the formation of volatile phenols, phenolic aldehydes, ketones, and acids, which upon further dehydration, are converted to lactones (Tarko et al., 2023).
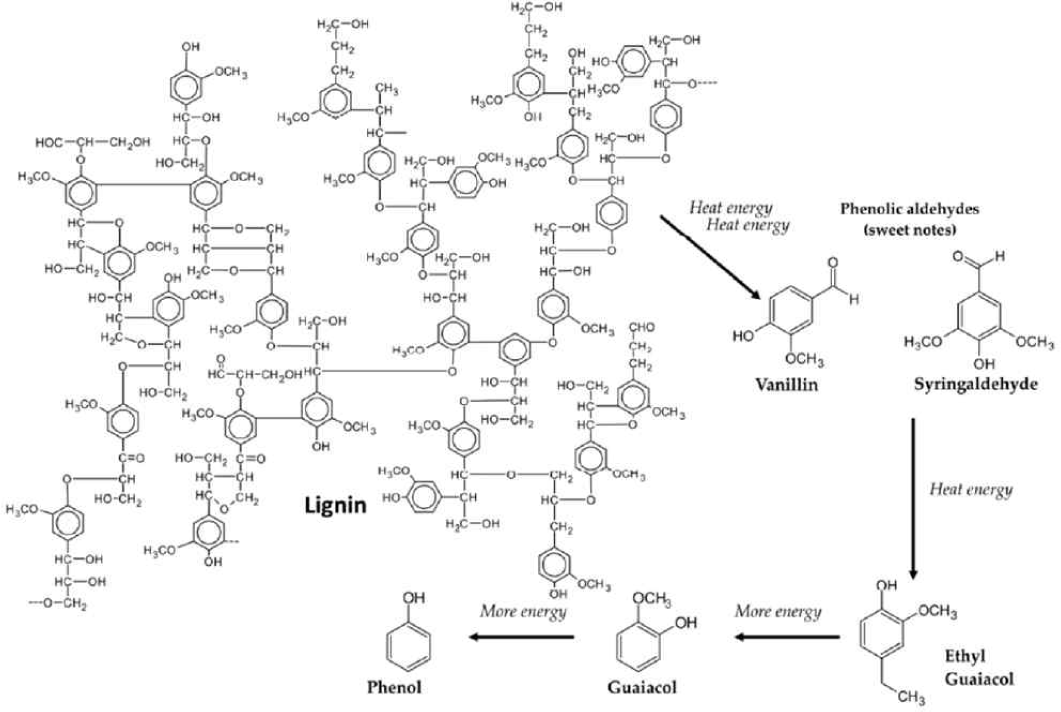
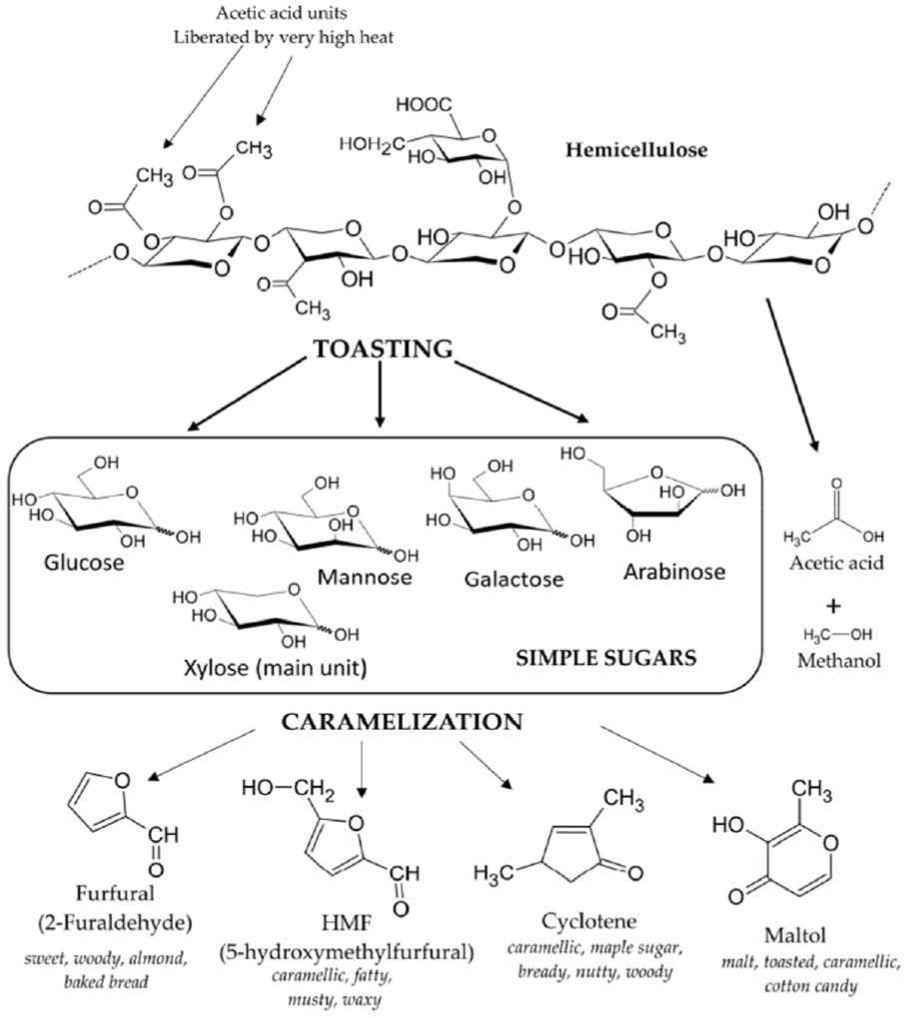
Toasting likewise causes the release of hemicellulose (simple sugars) leading to caramelization which gives the fruity, toasty, caramelized, and sweet aroma and flavors due to furfural, maltol, cyclotene and other sugar condensation products (Čabalová et al., 2018; González-Centeno et al., 2016; Nishimura, 1983; Prida and Chatonnet, 2010; Tarko et al., 2023). As presented in Fig. 7, results showed that the cellulose content decreases as the temperature increases due to thermal degradation of cellulose at higher temperatures.
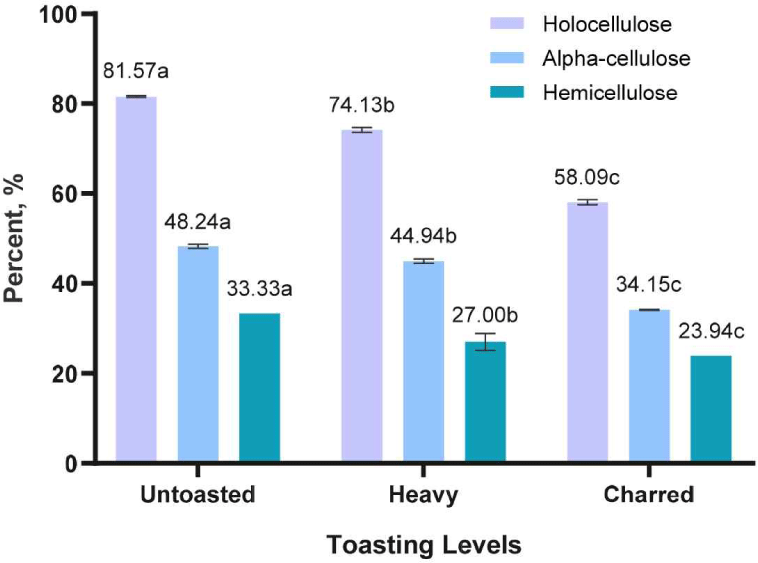
Cellulose binds the wood together. However, during barrel aging, it does not directly affect the maturation of alcoholic beverages, but serves as a transporter of extractives from wood to the alcoholic beverages (Aylott, 2003; Spedding, 2018). The toasting treatment could affect the physico-mechanical attributes of wood or the wooden barrels. Further studies must be made for verification.
The hemicellulose content of different toasted RRG wood was also determined. This hemicellulose is mainly consists of pentoses (xylose and arabinose), hexoses (mannose, glucose, and galactose), and uronic acids (d-Glucuronic acid and d-Galacturonic acid).
As presented in Fig. 7, hemicellulose content significantly decreases as the temperature increases. It is known that amorphous cellulose is less thermally stable than crystalline cellulose which are susceptible to toasting (Mburu et al., 2008; Özgenç et al., 2017). It was also observed by Sikora et al. (2018) that mannose, galactose and arabinose decrease upon increasing the treatment temperature.
As presented in Fig. 8, the lignin content significantly reduced in heavy toasted RRG samples due to thermal degradation. However, the charred RRG exhibited a greater lignin concentration than anticipated. This is possibly due to the reaction between lignin and products of polysaccharide degradation during the extraction process forming an insoluble fraction (Borrega et al., 2011; Overend and Chornet, 1987).
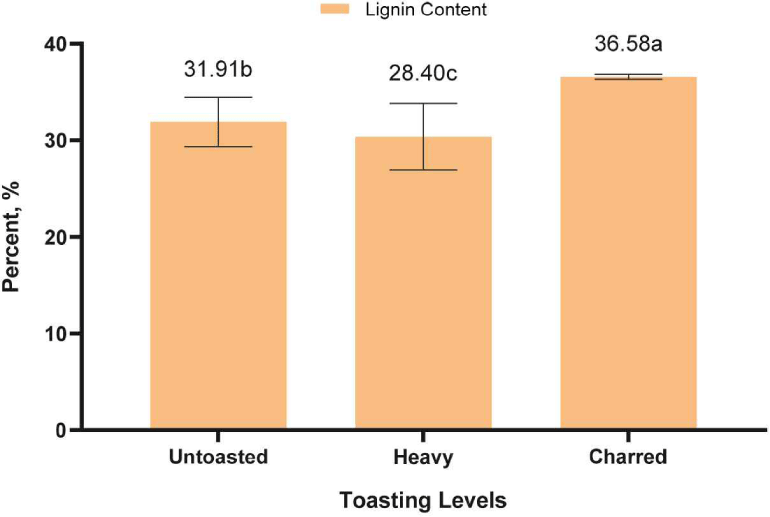
Future studies will conduct acid soluble lignin determination to verify the hypothesis that at higher temperatures, lignin polymer are degrades to form new compounds. Overall, toasting treatments could significantly degrade the macromolecular components of RRG wood as well as minor components such as extractives.
As mentioned, temperature increase breaks down lignin into new phenolic compounds (Hale et al., 1999; Tarko et al., 2023). Studies showed that ellagitannin, which is abundant in wood, is degraded and converted into ellagic acid during toasting (Cadahía et al., 2001; Jordão et al., 2012; Tarko et al., 2023). This is the main reason for the observed increase in TPC after heavy toasting, as shown in Fig. 9. Subsequently, the decline in TPC in charred RRG wood is due to the intense toasting temperature that resulted in the degradation of these phenolic compounds.
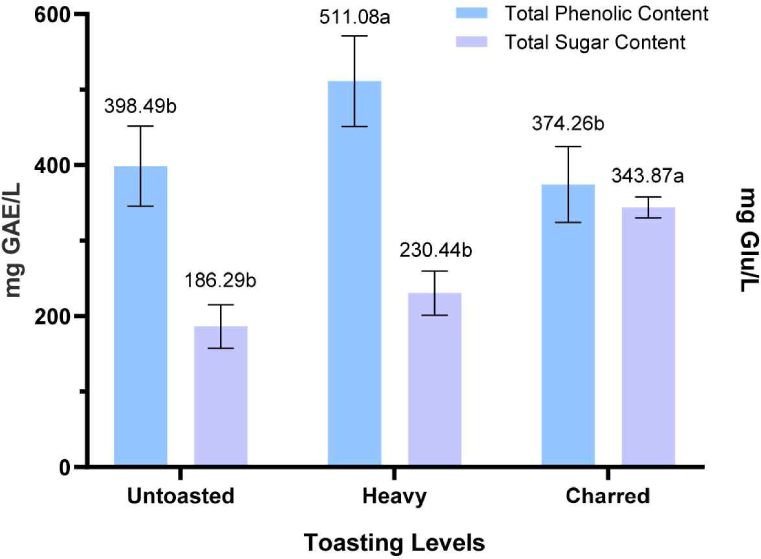
Additionally, the interaction of the extracted phenolic compounds in the solution may result in polymerization, leading to the formation of new compounds that could ultimately reduce the phenolic content of the extracts (Ivanova et al., 2012).
The direct relation of total sugar increase and temperature rise is due to the breakdown of hemicellulose into simpler sugars (Fig. 6). This is correlated with the decrease of hemicellulose content in the heavily toasted and charred RRG wood samples as previously presented (Fig. 7).
The increase in extracted sugars, primarily attributed to the breakdown of hemicelluloses through heat, was also observed in the research conducted by Kainuma et al. (2024) with the effect of toasting temperature on French oak wood chips in extracted sugars. At higher temperatures (> 250°C), sugars are transformed into several aromatic compounds, such as furfural and 5-methyl furfural, resulting in reduced extracted sugars (Le Floch et al., 2015). Kainuma et al. (2024) found that these results indicate two possible scenarios on the effect of heat treatment on hemicellulose: it becomes more soluble when depolymerized by heat, and is transformed into new aromatic compounds. Therefore, toasting temperature considerably influences the quantity, content, and composition of extracted sugars (Kainuma et al., 2024). Further studies will measure these simple sugars by HPLC analysis.
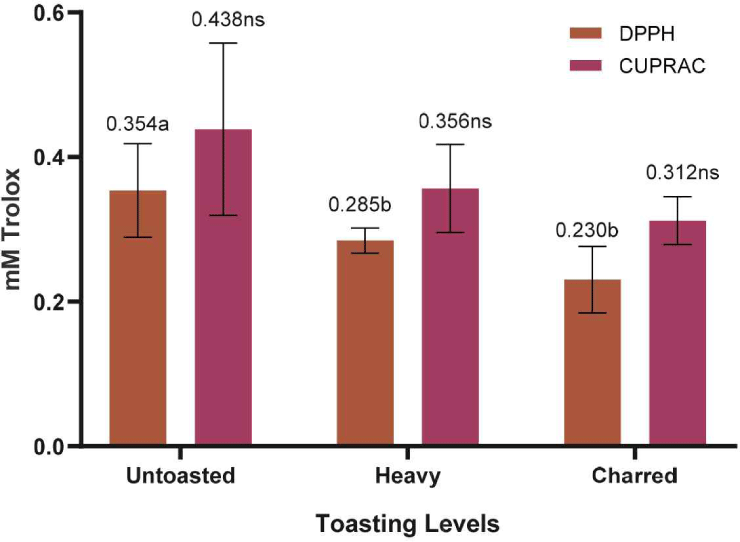
Furthermore, as shown in Fig. 10, the toasting of the RRG wood samples led to a decrease in antioxidant activity. However, a significant difference in antioxidant activity was observed only in the DPPH assays of the RRG wood samples. This decrease was due to the degradation of some phenolic and bioactive compounds which are known to contribute to the antioxidant activity.
As presented in Fig. 11, the toasting of RRG wood samples led to changes in the intensity of the bands in FTIR spectra. The band at 1,730–1,732 cm–1 increased after heavy toasting of RRG wood sample and decreased upon charring treatment. It has been reported that this band corresponds to C = O stretching vibrations of the acetyl groups of galactoglucomannan, carboxyl- and aldehydes, and aromatic/conjugated aldehydes and esters (Gérardin et al., 2007; Tjeerdsma and Militz, 2005).
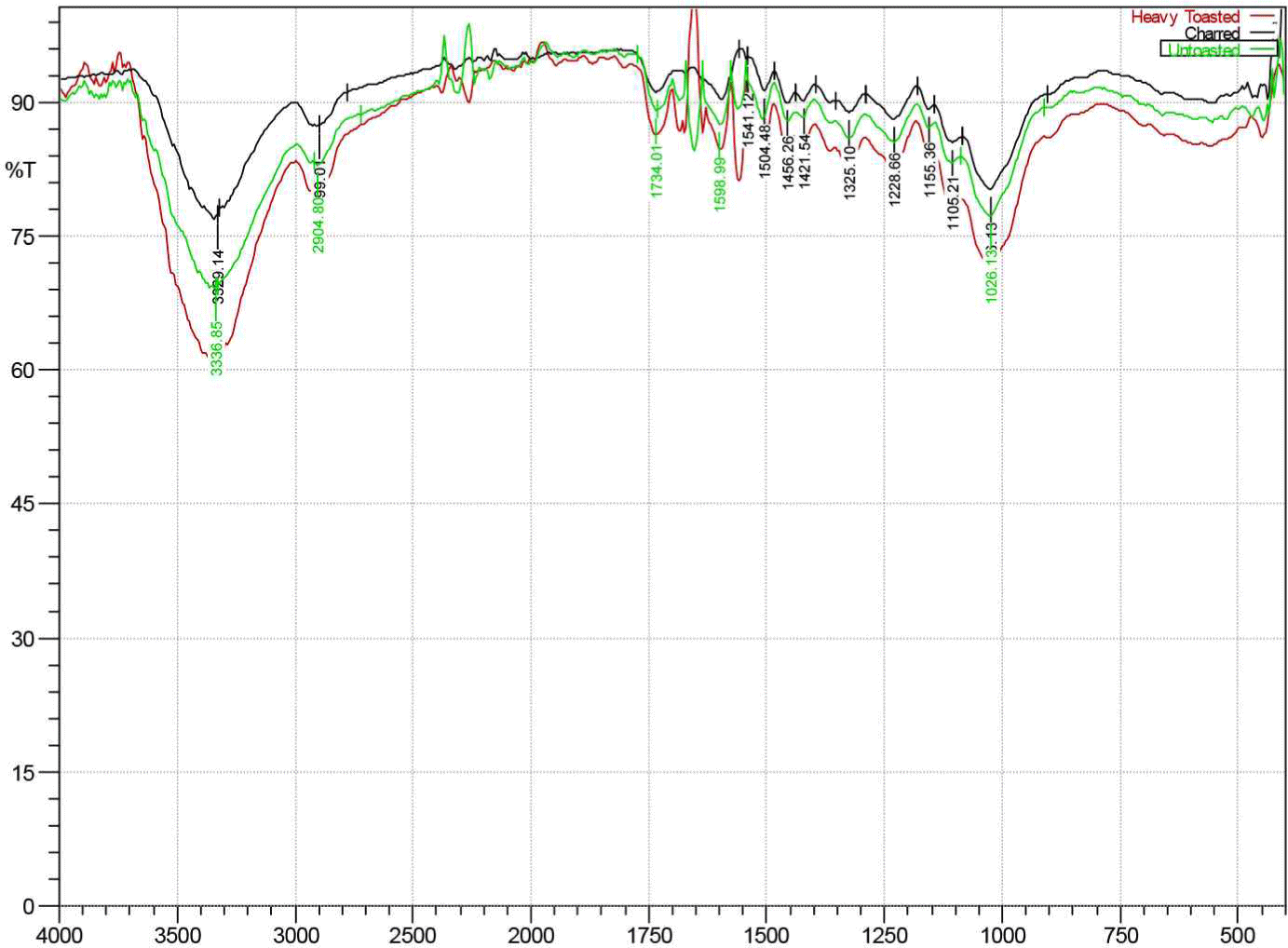
Thus, the heavy toasting treatment made the hemicellulose component of RRG wood partly soluble in hot water while charring treatment made it degraded, confirming previous results (Fig. 7). Furthermore, the degradation of hemicelluloses leads to the decrease of numerous free hydroxyl groups in hemicelluloses which correlates to the decrease of the band intensity at 1,650–1,652 cm–1 (Akgül et al., 2007; Gérardin et al., 2007).
As also observed in Fig. 11, the bands’ intensities at 1,504–1,508 cm–1, 1,452–1,459 cm–1, and 1,421–1,422 cm–1 increase upon toasting the RRG wood samples heavily compared to untoasted RRG wood samples. This could be due to condensation of lignin and splitting of aliphatic side chains in lignin during heating (Akgül et al., 2007; Kotilainen et al., 2000; Schulz et al., 2021). These changes in lignin during heat treatment were also reported in Eucalyptus grandis and Pinus elliottii wood (Gallio et al., 2019; Schulz et al., 2021). These bands also represent the C = C stretching of the aromatic skeletal vibrations which also represent the lignin content changes in heat-treated samples (Özgenç et al., 2017).
Overall, the FTIR results confirm the degradation of lignin and hemicellulose of RRG wood samples during heat treatment.
Ethanolic extracts from untoasted, heavily toasted and charred RRG wood were further subjected to GC-MS analysis to determine the volatile compositions which could give aroma to alcoholic beverages. As observed in Table 4, the main components of untoasted RRG wood ethanolic extract were furfural and eugenol with 39.18% and 26.22%, respectively. On the other hand, the main composition of ethanolic extract from heavy toasted RRG wood were 3,5-dimethoxy-4-hydroxycinnamaldehyde (28.76%), coniferyl aldehyde (28.10%), syringaldehyde (14.25%) and vanillin (8.16%). Lastly, the main components of ethanolic extract from charred RRG wood were 3,5-Dimethoxy-4-hydroxycinnamaldehyde (28.58%), syringaldehyde (21.50%), coniferyl aldehyde (11.43%), vanillin (5.99%) and syringylacetone (5.90%).
The results also showed that the toasting process and the increase in thermal temperature produce a greater number of volatile components from wood. Specifically, the heavily toasted RRG and charred RRG samples yielded 34 and 48 volatile components respectively, as shown in Tables 5 and 6, in contrast to the 11 volatile components seen in the untoasted RRG wood sample (Table 4). Furthermore, the main volatile components (coniferyl alcohol, syringaldehyde and vanillin compounds) of heavy and charred RRG wood extracts came from the degradation of lignin due to thermal treatment. They are known for their aromatic and sweet notes. Additionally, the dehydration of hemicellulose during aging leads to the formation of new compounds such as furfural and 5-hydroxymethylfurfural, which could provide roasted, fudge, and caramel profiles (Kelly et al., 2023). These compounds are commonly found in alcoholic beverages aged in oak barrels and wood chips.
Overall, E. camaldulensis wood and toasting treatment could also significantly improve the sensory profile of alcoholic beverages. Moreover, the locally available and fast-growing wood species will serve a sustainable alternative in the Philippine alcoholic beverage industry.
4. CONCLUSIONS
The toasting treatment had a significant effect on the macromolecules and extractives of RRG wood. Furthermore, GC-MS results showed that the degradation of these macromolecules leads to the formation of new compounds which could improve the sensory profile of alcoholic beverages. Future studies will focus on the quantitative determination of individual lignin-derived and hydrolyzed hemicellulose compounds through HPLC and GC analysis.

