1. INTRODUCTION
The use of wood as a valuable material is determined by physical or chemical properties. Wood is primarily composed of cellulose, hemicellulose, lignin, and extractive, which are linked together by various chemical bonds such as hydrogen and carbon-carbon (Yeon et al., 2019). Acacia mangium is one of the Acacia species commonly used in Indonesia as in Indonesia for pulp and paper production. This is due to the faster growth, good adaptability, and satisfactory pulp yield. Fast-growing tree species, such as A. mangium are generally planted to achieve short felling rotations (typically from 6 to 10 years; Hadi et al., 2020).
Over the past decade, extensive regions of Acacia plantations in Sabah (Malaysia) and Sumatra (Indonesia) have been severely damaged or destroyed by fungal diseases, particularly root rot and stem wilt canker caused mainly by Ganoderma spp. and Ceratocystis spp. respectively (Tarigan et al., 2011; Wingfield et al., 2011). These diseases caused a 40%–60% reduction in A. mangium productivity (Nambiar et al., 2018). The widespread damage caused by these diseases prompted plantation managers to replace A. mangium plantations with Eucalyptus pellita. However, many cases of damping-off were found in the nursery of Eucalyptus sp. (Nair, 2000). Acacia auriculiformis is also a less preferred species for planting in production forests and more resistant to heart rot (Barry et al., 2005). Although heart rot does not kill the tree, it reduces the volume of marketable wood by up to 24% (Sudin et al., 1993). Mihara et al. (2005) reported that the high content of flavonoids, namely the compounds of 3,40,7,8-tetrahydroxyflavanone and teracacidin in the heartwood of A. auriculiformis plays a very important role in resistance to heart rot disease. Furthermore, these flavonoids showed strong antioxidant activity and laccase inhibition, suggesting they may combat fungal growth by scavenging free radicals produced by fungal laccase enzymes. On the other hand, Barry et al. (2005) also stated that three types of flavonoids such as 2,3 trans-3,4’,7,8-tetrahydroxyflavanone, teracacidin, and 4’,7,8,-trihydroxyflavanone in A. auriculiformis heartwood play a role against heart rot fungal disease.
In 2008, the Indonesian Forestry Instrument Standard Assessment Centre started developing an Acacia hybrid breeding strategy through a controlled cross-pollination technique between A. mangium and A. auriculiformis as well as increasingly intensive clone planting. Previous studies have reported that Acacia hybrid shows better growth, suitable for pulp and paper, adaptive to various soil types, and even produces more pulp (Yamada et al., 1992). In addition, Acacia hybrid is also more resistant to diseases (Kim et al., 2008).
The composition of wood extracts includes primary metabolites, which are essential for growth and cellular functions (Mai and Zhang, 2023) and secondary metabolites, which are derived from primary metabolites and dominate the extractive content, particularly during heartwood formation (Spicer, 2005). Wood extracts are rich in complex organic compounds, including polyphenols, flavonoids, and terpenoids, which demonstrate diverse biological activities. Previous studies have been investigating the bioactive properties and potential benefits of natural compounds found in plants, including their antioxidant, antimicrobial, and anti-inflammatory effects (Hidayat et al., 2018, 2019; Huh et al., 2022; Kuspradini et al., 2024; Manurung et al., 2019; Yang et al., 2019, 2022). In addition, these compounds play a key role in defending trees against biotic threats, such as termite damage and fungal infections (Adfa et al., 2023; Alfredsen et al., 2008; Kadir and Hassan, 2020; Nkogo et al., 2022; Oramahi et al., 2022; Suprianto et al., 2023; Zalsabila et al., 2024). Furthermore, extractives offer protection against abiotic stressors, including UV radiation and oxidative damage, thereby enhancing the durability of wood (Anish et al., 2023).
Extractives in the wood industry serve a dual purpose: they enhance the durability of wood by providing effective preservation and photo-stabilization, thereby improving resistance to decay and photodegradation (Gao et al., 2024). Several specific extractive compounds from heartwood show promise as natural biocides against fungal infections and termite infestations, including quinone compounds from Tectona grandis wood, rubrynolide compounds from Sextonia rubra wood, and n-hexane extracts from Dalbergia congestiflora wood (Dungani et al., 2012; Martinez-Sotres et al., 2012; Rodrigues et al., 2011). Additionally, the natural pigments in extractives make them an attractive option for coloring fast-growing wood, imparting a unique aesthetic value to the final product (Gao et al., 2024). Natural pigments or natural dyes are generally derived from the tannin group found in wood extracts. Previous studies have identified several wood species as sources of tannins or neutral extracts for natural dyes, including merbau wood and Pinus radiata bark (Mindaryani et al., 2023; Mun et al., 2020, 2021).
In general, extractive include a variety of chemical compounds soluble in polar or non-polar solvents and are present in all morphological wood regions including parenchyma, vessels, fibers, cell lumens, and cell wall capillaries. However, the xylem content is higher in heartwood (Hillis, 1971, 1987). The composition and distribution of extractive vary according to species, growing location, as well as position within the tree, and genetic factors (Hillis, 1987). Extractive contributes to the color and natural durability of the heartwood. On the other hand, the influence of both non-polar and polar extractives result in higher chemical consumption during pulping and bleaching, increased production costs, a greater occurrence of quality defects, and elevated operating expenses in the pulping process (del Rio et al., 1998; Hillis and Sumimoto, 1989). Small amounts of lipophilic wood extractives can cause the formation and deposition of pitch on pulp and equipment, which can affect the efficiency of wood processing in pulp and paper production (Back and Allen, 2000).
Natural resistance is associated with the extractive composition in the heartwood, primarily phenolics, which are the major components and have antioxidant capabilities (Rababah et al., 2011). Several phenolic compounds such as teracacidin and 3,4,7,8-tetra-hydroxy flavanone were observed abundantly in wood extracts of A. mangium and A. auriculiformis which showed antifungal activity (Barry et al., 2005; Pietarinen et al., 2004). Mihara et al. (2005) also reported that these compounds can fight against heart rot disease associated with the scavenging of hydroxyl radicals created by the fungus, as a correlation between antioxidant activity, laccase inhibition, and antifungal activity.
Regarding information on wood properties, several studies have been conducted on Acacia hybrid, such as in Malaysia (Muhammad et al., 2017), Vietnam (Kim et al., 2008), and India (Sharma et al., 2016). However, reports on the basic properties of Acacia hybrid wood in Indonesia are still very limited, specifically those derived from clones. There is limited information on the anatomical properties and cell wall components of Acacia hybrid produced from open cross-pollination without information on the male parent (Nirsatmanto et al., 2017; Yahya et al., 2010). Previous studies only focused on the general wood extractive and lipophilic compounds (Soon and Chiang, 2012; Yahya et al., 2010). However, studies on the relationship between wood extractives, antioxidant activity, and wood resistance in different radial positions are still limited (Purba et al., 2021). Hence, the aim of this study was to evaluate extractive composition and antioxidant activity of Acacia hybrid variety Purwo Sri Ah015D in axial and radial positions to examine the potential for natural durability for pulp materials or even the benefits in other fields such as pharmacology.
2. MATERIALS and METHODS
Wood samples (10 years) were obtained from Acacia hybrid (A. mangium × A. auriculiformis) variety, namely the variety Purwo Sri Ah015D registered for Plant Variety Protection Number: 949/PVHP/2022. Wood of the variety was planted in clonal trial in Wonogiri (7°47’S, 110°56’E), Central Java, Indonesia. The environmental conditions of the sample are as follows: an elevation of 141 meters above sea level, vertisol soil, flat topography, a mean monthly temperature of 26.4°C, and an annual rainfall of 2,750 mm. The samples were felled during the dry season, which is expected to minimize external influences on the sampling process. This variety was the result of crossing parental family 86 (provenance Claudie River, Aust) × family 107 (provenance Melville Int Orchard, Queensland). The hybrid parent was selected from plus trees in the first-generation progeny trial, and then tested in the clonal trial established to verify the growth and site adaptability.
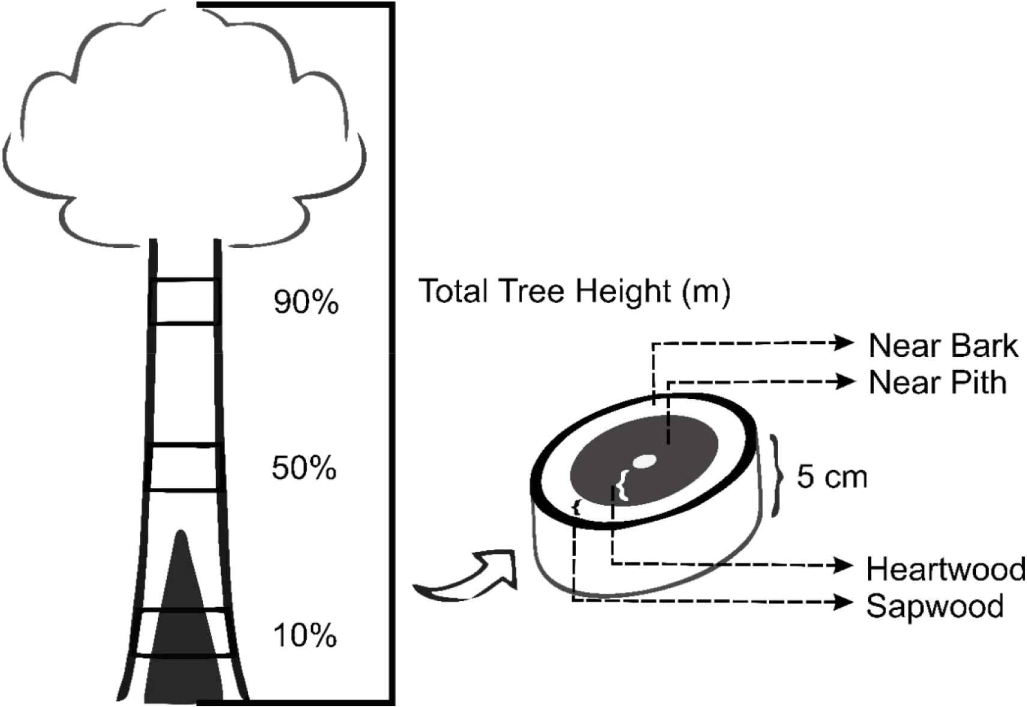
Three trees of the Purwo Sri Ah015D variety from the clonal trial were felled. Wood discs (5 cm thick) were taken from the bottom (10%), middle (50%), and top part (90%) of the total merchantable tree height, where the tree diameter was greater than 4 cm (Fig. 1). This criterion was selected to capture the variation in extractive content along the longitudinal axis. Research by Iswanto et al. (2021) highlights the crucial role of axial position in determining the diversity of chemical components in wood. Arisandi et al. (2021) also found that extractive levels decrease from the bottom to the top of the tree. The heartwood proportion in the bottom part varied between 11.8% and 26.5%. The tree height of Acacia hybrid ranged from 10.8 to 16.3 m and the disc thickness for the bottom, middle, and top ranged from 6.25 to 8.25, 6.05 to 6.90, 4.45 to 5.35 cm, respectively. For analysis of extractive content, wood of each disc was drilled in near bark (1.5 cm from the bark) and near pith (1 cm from the pith) in four cardinal directions which were then mixed (in each part) to avoid radial variations. The powder was ground and sieved until it passed 40–60 mesh which was used for analysis.
Chemicals such as sodium carbonate, aluminum chloride, sodium hydroxide, and Folin-Ciocalteu’s phenol were obtained from Merck (Darmstadt, Germany). Meanwhile, gallic acid, quercetin, D-glucose and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were acquired from Sigma-Aldrich (St. Louis, MO, USA) and catechin was acquired from Shanghai Chemical Industry (Shanghai, China).
About 5 grams of powder were extracted successively with n-hexane and methanol for 6 hours in a Soxhlet apparatus, followed by hot water reflux extraction for 3 hours. The solvent was removed using the rotary evaporator, and the weight of the extract is expressed as a percentage of dry wood. Dalimunthe et al. (2022) stated that the Soxhlet and reflux extraction methods were chosen for their effectiveness in extracting phenolic and flavonoid compounds with high antioxidant activity, compared to cold water extraction methods, such as maceration and percolation. Methanol and hot water extracts were used for analysis of total phenolic content (TPC), total flavonoid content (TFC), total flavanol content (TVC), total soluble polysaccharide content (TSP), and antioxidant activity (methanol extract). Methanol and hot water solvents or their combinations are most frequently used to extract phenolic compounds from several plant sources. Kapasakalidis et al. (2006) stated that methanol is widely recognized as the most effective solvent for polyphenol extraction. Furthermore, Arab et al. (2011) found that methanol demonstrated substantially greater antioxidant activity. Unlike phenolic compounds, n-hexane solvents are particularly effective at dissolving non-polar substances such as fats, waxes, resins, and sterols (Lukmandaru, 2011).
TPC was assessed using the method of Folin-Ciocalteu (Singleton et al., 1999). About 2.5 mL of diluted Folin-Ciocalteu phenol reagent as well as an aqueous solution (1:9, v/v) were mixed with 0.5 mL of the sample extract (0.25 mg/mL) in a 9 mL glass. After an interval of 2 minutes, 2 mL of 7.5% aqueous sodium carbonate (Na2CO3) was added to the glass, the mix was maintained at ambient temperature for 30 minutes. The absorbance of the standard solutions was measured at 765 nm against a blank using a Visible spectrophotometer (VIS-WPA S800+, Visible, Biochrom, Holliston, USA). Furthermore, TPC was measured as the average ± SD calculated from three replicate trees and reported as milligrams of gallic acid equivalents (mg GAE/g dried extract).
TFC was determined using the AlCl3 method (Brighente et al., 2007). About 2 mL of the sample at 1 mg/mL concentration was added to 2% AlCl3·6H2O solution and incubated for 1 hour at 20°C. The absorbance for the standard solution was tested against the blank at 415 nm using a Visible spectrophotometer (VIS-WPA S800+, Visible). Furthermore, TFC was measured as the mean ± SD of three tree replication measurements and was expressed in milligrams of quercetin equivalents (mg QE/ extract).
TVC was measured using the vanillin HCl assay, following the method described by Miranda et al. (2016) with modifications. About 0.5 mL (0.25 mg/mL) of the sample solution, 3 mL vanillin reagent (4% vanillin methanol), as well as 1.5 mL HCl were added, then the reaction was left to stand for 15 min at ambient temperature. The standard solution absorbance was recorded at 500 nm using a Visible spectrophotometer (VIS-WPA S800+, Visible) with a blank for calibration. TVC was calculated as the average ± SD from measurements of three trees and expressed in milligrams of catechin equivalents (mg CE/g dried extract).
TSP were measured according to DuBois et al. (1956). Approximately 1 mL of the sample solution (0.25 mg/mL) was combined with 1 mL of 5% phenol reagent solution (5 g in 100 mL distilled water), followed by the addition of 5 mL of sulfuric acid (98%). The mix was allowed to sit at room temperature for 20 minutes, and its absorbance was measured at 490 nm utilizing a Visible spectrophotometer (VIS-WPA S800+, Visible) against a blank. The TSP value was presented as the average ± SD based on three tree replicates, expressed in milligrams of glucose equivalents (mg GE/g dried extract).
Approximately 0.1 mL of the methanol extract in some variation concentrations of 62.5, 125, 250, and 500 μg/mL was included to the arrangement of 3 mL of 0.1 mM DPPH in methanol. The solution was shaken and kept in the dark at 22°C for 30 min. Using the same procedure, the blank solution was also reacted without the extract. Next, the absorbance of the sample was assessed at 512 nm. Inhibition was calculated according to following Equation (1):
Where A0 represents the blank absorbance and A1 represents the absorbance for the extracted. Antioxidant activity was expressed as IC50. In this experiment, all analyses were performed in three replicate tree measurements. Furthermore, gallic acid and quercetin were used as positive controls.
The data were analyzed statistically utilizing SPSS software (version 25, IBM, New York, NY, USA). Analysis of variance (ANOVA) was performed, with significance determined at the 95% confidence level. Two-way ANOVA was used to evaluate the influence of axial position (bottom, middle, and top) and radial (near pith and near bark) positions on extractive content, TFC, TPC, TVC, and TSP, and antioxidant activity. Subsequently, Duncan’s test was conducted to identify differences between groups. In addition, Pearson correlation analysis was performed to assess the correlations between extractive levels and phenolic groups, as well as antioxidant activity.
3. RESULTS and DISCUSSION
Lipophilic extractive including fats, waxes, resins, and sterols dissolve in non-polar solvents such as n-hexane solution (Lukmandaru, 2011). Meanwhile, phenolic and sugar compounds are dissolved by polar solvents such as methanol and hot water (Fengel and Wegener, 1989; Han and Rowell, 1997). The result showed that the mean content of n-hexane, methanol, and hot water extractive in the oven-dried wood of the Acacia hybrid Variety Purwo Sri Ah015D ranged from 0.36% to 0.73%, 0.94% to 1.63% and 0.58% to 0.82%, respectively (Table 1). These values were lower than those reported in previous studies on a 6-year-old Acacia hybrid from Indonesia (Purba et al., 2021) and a 4-year-old sample from Vietnam (Kha, 2000). The results were also smaller compared to the species of A. mangium and A. auriculiformis aged 4–6 years for both non-polar and polar solvents (Kha, 2000; Lange and Hashim 2001; Mihara et al., 2005; Muladi et al., 2003). Although the samples used were older (10 years old), the levels of both polar and non-polar extractive were not higher than in previous studies. Furthermore, this study also found that the extractive content was lower compared to other Acacia species, such as A. confusa, A. dealbata, A. mearnsii, A. nilotica, A. crassicarpa, A. leucophloea, and A. decurrens, in several parts, including wood, heartwood, bark, twigs, and leaves (Chang et al., 2021; Correia et al., 2022; Oliveira et al., 2020; Pravesh et al., 2017; Prayogo et al., 2021). In addition, the extractive content of n-hexane was also lower compared to other species, such as S. mahagoni, which ranged from 0.76% to 2.45% (Arisandi et al., 2024b). Similarly, the amount of methanol extract from keruing and meranti wood ranged from 1.97% to 3.83% (Fernandes et al., 2024). The percentage of heartwood in this sample was significantly lower (present only at the base), even though it was 10 years old. Interestingly, in the study by Purba et al. (2021) on Acacia hybrid clone samples (6 years) 16, 55, and 44 from the same location had a heartwood percentage ranging from 59.47% to 67.23%.
High heartwood generally has a significant content of extractive, including both phenolic and lipophilic extractive. Meanwhile, high levels of extractive, specifically non-polar ones which are usually dominated by lipophilic compounds, will have a negative impact on wood raw materials used for pulp and paper production. Even at low levels, lipophilic extractives in wood can reduce processing efficiency in pulp and paper production, causing pitch formation and deposition on both pulp as well as equipment (Back and Allen, 2000).
Methanol solvent dissolved 48.7%–60.4% of the total extracted material, while extractive dissolved in dichloromethane contributed a small percentage of the total. Polar solvents, such as methanol and hot water, accounted for approximately 75.2% to 86.2% of the total extractive (Fig. 2). The percentage composition of this polar solvent was slightly lower compared similar to findings in other species, such as E. globulus wood (Miranda and Pereira, 2002; Morais and Pereira, 2012). Differences are probably due to different tree ages, species, solvents and extraction methods (Freire et al., 2005; Mészáros et al., 2007; Pereira, 1988).
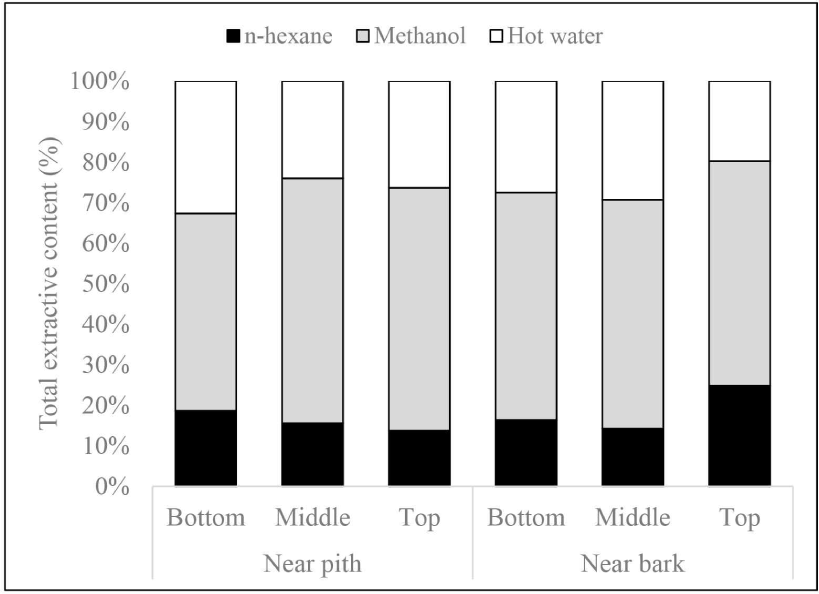
The two-way ANOVA test results indicated that only extractive content of n-hexane was significantly influenced by the interaction of radial and axial positions. Meanwhile, there was no significant effect of radial or axial position on the extractive content in methanol and hot water extracts (Table 2).
| Source of variation | df | n-Hexane | Methanol | Hot water |
|---|---|---|---|---|
| Radial (R) | 1 | ** | ns | ns |
| Axial (A) | 2 | ns | ns | ns |
| R × A | 2 | * | ns | ns |
| Error | 12 | |||
| Total | 17 |
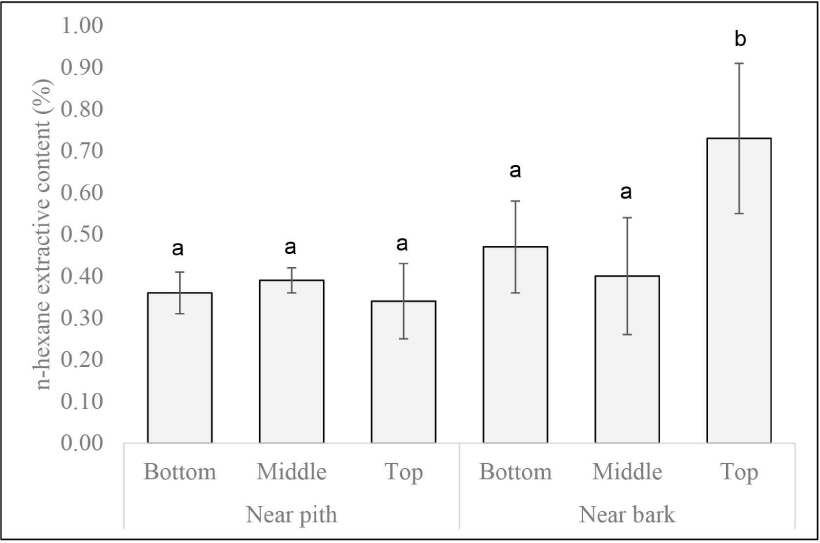
In general, extractive content was greater closer to the bark than near the pith (Fig. 3). The levels were found to be significantly different for extractive content of n-hexane at the top of the tree. This may be due to the proportion of young and mature wood. Haupt et al. (2003) found lower concentrations of wood extractive in teak (T. grandis) juvenile wood compared to mature heartwood, resulting in the lower natural durability of juvenile wood. Dünisch et al. (2010) also reported that the content of heartwood extractive in polar solvents, particularly methanol and mature heartwood showed higher acetone content (9.8%–10.3%) compared to juvenile (6.3%–7.7%). Furthermore, extractive content increased from the bottom to the top of the tree. Lukmandaru et al. (2021) stated that the high levels of extractive at the upper part are likely due to the position close to the crown as a place of photosynthesis. Yeh et al. (2006) reported that the levels of benzene alcohol extractive in Pinus taeda were found to be higher at the top than at the base parts. This is also likely because there are more living cells at the top of the tree than at the bottom part, where the levels of primary extractive such as sugar and fat are food reserves for metabolic processes. Notably, only the n-hexane extract near the bark shows a significant increase in value from the base to the top of the tree, likely due to the higher proportion of sapwood with increased fat content in this region.
TPC, TFC, TVC, and TSP in methanol extract ranged from 135.7 to 402.9 mg GAE/g dried extract, 9.55 to 29.6 mg QE/g dried extract, 12.2 to 31.7 mg CE/g dried extract, and 368.5 to 475.4 mg GE/g dried extract, respectively. Meanwhile, the levels in the hot water extract ranged from 50.3 to 81.0 mg GAE/g dried extract, 5.81 to 7.59 mg QE/g dried extract, 42.9 to 64.0 mg CE/g dried extract, 139.2 to 330.5 mg GE/g dried extract (Table 3). For TPC, the result was slightly lower compared to a previous study in Acacia hybrid heartwood which ranged from 76.8 to 448.3 mg GAE/ g in methanol dried extract and 43.2 to 198.9 mg GAE/ g in hot water dried extract (Purba et al., 2021). Compared to other species, TPC in this study was lower than A. mangium (28.5% to 31.7%), A. auriculiformis (43.3% to 76.6%), A. confusa heartwood (535.2 mg GAE/g) and A. dealbata twigs (351.3 mg GAE/g) in acetone and methanol extract (Barry et al., 2005; Chang et al., 2021; Correia et al., 2022). Moreover, the level in E. pellita wood ranged from 368.4 to 632.5 in ethanol as well as 199.9 to 429.6 mg GAE/g dried extract in hot water (Arisandi et al., 2019). The TPC in E. urograndis, E. grandis, and E. maidenii ranged from 687.89 to 825.47 mg GAE/g dried extract in methanolic or hot water extract (Santos et al., 2013). However, the results in this study were higher than those found in the heartwood of A. nilotica, A. leucophloea, and A. catechu, which ranged from 6.2 to 10.86 mg GAE/g dried extract in methanol, ethanol, and hot water extracts (Sulaiman et al., 2014). A similar trend was also found in A. nilotica, A. leucophloea, A. crassicarpa, and A. decurrens heartwood and bark, with values ranging from 19.6 to 259.0 mg GAE/g in methanol and hot water extracts (Pravesh et al., 2017; Prayogo et al., 2021), and in E. camaldulensis, E. globulus, and E. rudis, with values ranging from 5.22 to 25.63 mg GAE/g dried extract (Conde et al., 1995).
The levels of flavonoid content in this study were slightly lower than previous studies in sapwood and heartwood of Acacia hybrid, which ranged from 29.5 to 133 mg QAE/ g in methanol dried extract and 7.70 to 29.3 mg QAE/ g in hot water dried extract (Purba et al., 2021). However, the level was lower than that of A. confusa (216 to 252.3 mg RE/g dried acetone and toluene/ethanol extract), A. dealbata (87.0 mg CatE/g dried methanol extract), and A. crassicarpa (65.2 mg QE/g dried methanol extract; Chang et al., 2021; Correia et al., 2022; Prayogo et al., 2021). On the other hand, this study had higher levels compared to the heartwoods of A. nilotica, A. leucophloea, and A. catechu, with values ranging from 2.2 to 18.9 mg QE/g in methanol and hot water extracts (Pravesh et al., 2017; Sulaiman et al., 2014). TVC in this study were lower than those in sapwood and heartwood of Acacia hybrid in methanol extract (83.3 to 218.2 mg QE/g dried extract), but higher than those in hot water extract (7.08 to 25.3 mg CAE/g dried extract; Purba et al., 2021). Arisandi et al. (2019) also reported that the levels of flavanol in E. pellita wood ranged from 165.6 to 373.8 as well as 47.5 to 132.5 mg CE/g dried extract in ethanol and hot water extract, respectively. On the other hand, TVC of this study was also higher than that of A. mangium and A. auriculiformis heartwood which ranged from 0.77% to 2.56% (Barry et al., 2005) and in Eucalyptus globulus species (13.93 to 17.0 mg GAE/g dried extract; Luís et al., 2014). Differences in TPC, TFC, and TVC content can be attributed to variations in age, tree species, diameter range, and location. For example, Purba et al. (2021) reported the TPC, TFC, and TVC in younger Acacia hybrid (6 years), but with a greater breast height diameter (19.25 to 30.25), compared to this study, where the diameter was between 4.45–8.25 cm from top to bottom part.
TSP was in accordance with previous studies in other species. Arisandi et al. (2021) reported that TSP in Swietenia mahagoni was 440.4 mg GE/ g dried hot water extract. However, the values obtained in this study were higher than those reported for Melaleuca cajuputi wood and bark which ranged from 79.3 to 102.8 as well as 148.8 to 165.9 mg Glu/g dried extract in methanol and hot water respectively (Arisandi et al., 2024a). The TSP content in Swietenia macrophylla was lower, with inner bark ranging from 91.5 to 97.3 mg Glu/g dried extract and outer bark ranging from 78.7 to 148.2 mg Glu/g dried extract in methanol and hot water extract, respectively (Masendra et al., 2020).
In general, methanol dissolves the highest levels of TPC, TFC, and TSP compared to hot water solvents. The reverse pattern was found for the TVC concentrations, which were more dissolved by hot water solutions. Methanol solvent is very effective in dissolving antioxidants, particularly phenolic compounds, which are the primary contributors. Most of these compounds are classified as hydrophilic antioxidants (Huang et al., 2002). The levels of TPC, TFC, TVC, and TSP, which are generally soluble in polar solvents, are not significantly different from previous studies (Table 3).
The two-way ANOVA analysis results indicated that the TPC value of wood from Acacia hybrid Variety Purwo Sri Ah015D was significantly influenced through the interaction of axial and radial positions. The difference in axial position had a significant effect on the concentration of TFC, TVC, and TSP (Table 4). In general, TPC was higher near the pith than near the bark, while TPC at the bottom part (methanol-soluble extract) and top (hot water-soluble extract) was significantly higher near the pith than near the bark [Fig. 4(a) and (b)]. This trend might be related to the presence of heartwood near the pith. As stated by the IAWA (1964), heartwood in growing trees no longer contains living cells, as reserve materials, including starch, are converted into heartwood substances such as phenolic compounds.
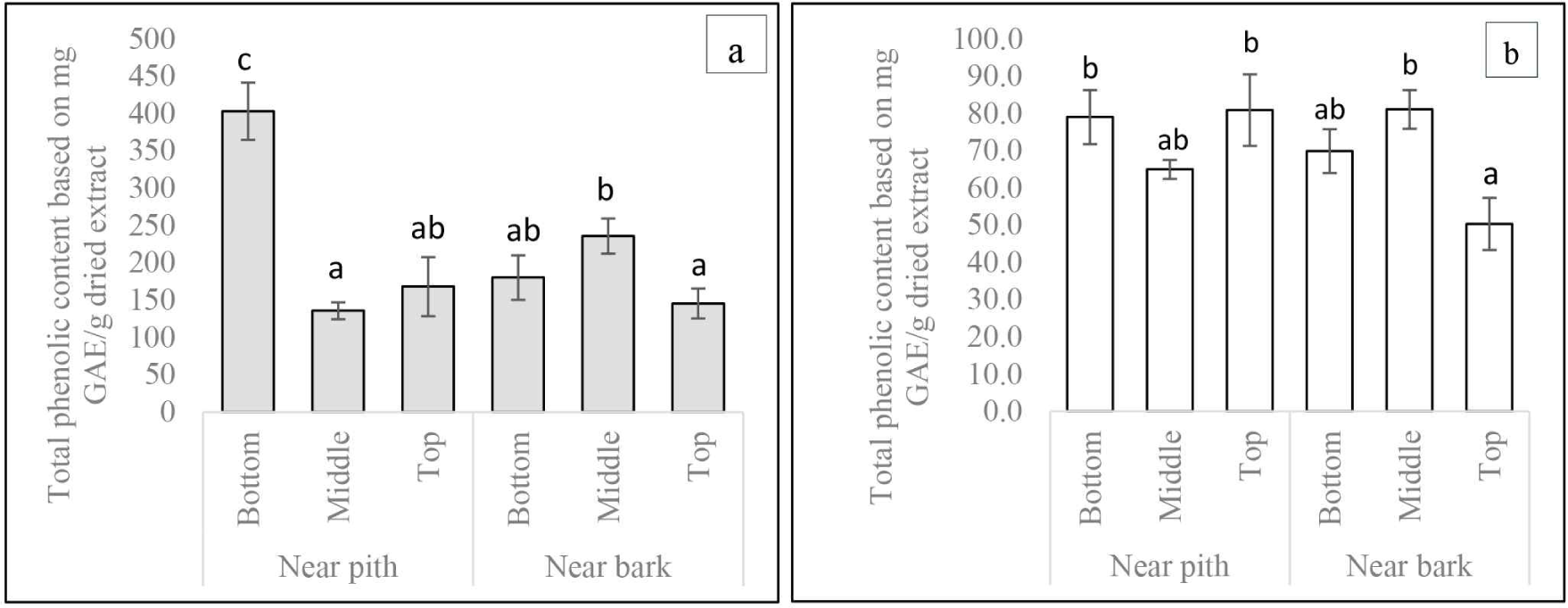
The concentration of TPC declined along the tree’s height about axial position differences. TPC was significantly higher at the base of the tree compared to the middle and the top [Fig. 4(b)]. This may also be explained by the heartwood content, which is generally higher at the base compared to the middle and top parts. The pattern was consistent with that of previous studies on several species, including S. mahagoni and Larix sibirica (Arisandi et al., 2021; Neverova et al., 2013). Furthermore, the concentrations of TFC and TSP showed a significant decline along the tree’s height [Fig. 5(a) and (c)]. The reverse pattern was found in TVC levels [Fig. 5(b)]. This shows that the distribution of flavonoid compounds which are part of polyphenols was not all concentrated at the bottom, but also at the top similar to the TVC pattern. Therefore, the top part from Acacia hybrid Variety Purwo Sri Ah015D also has high antioxidant potential.
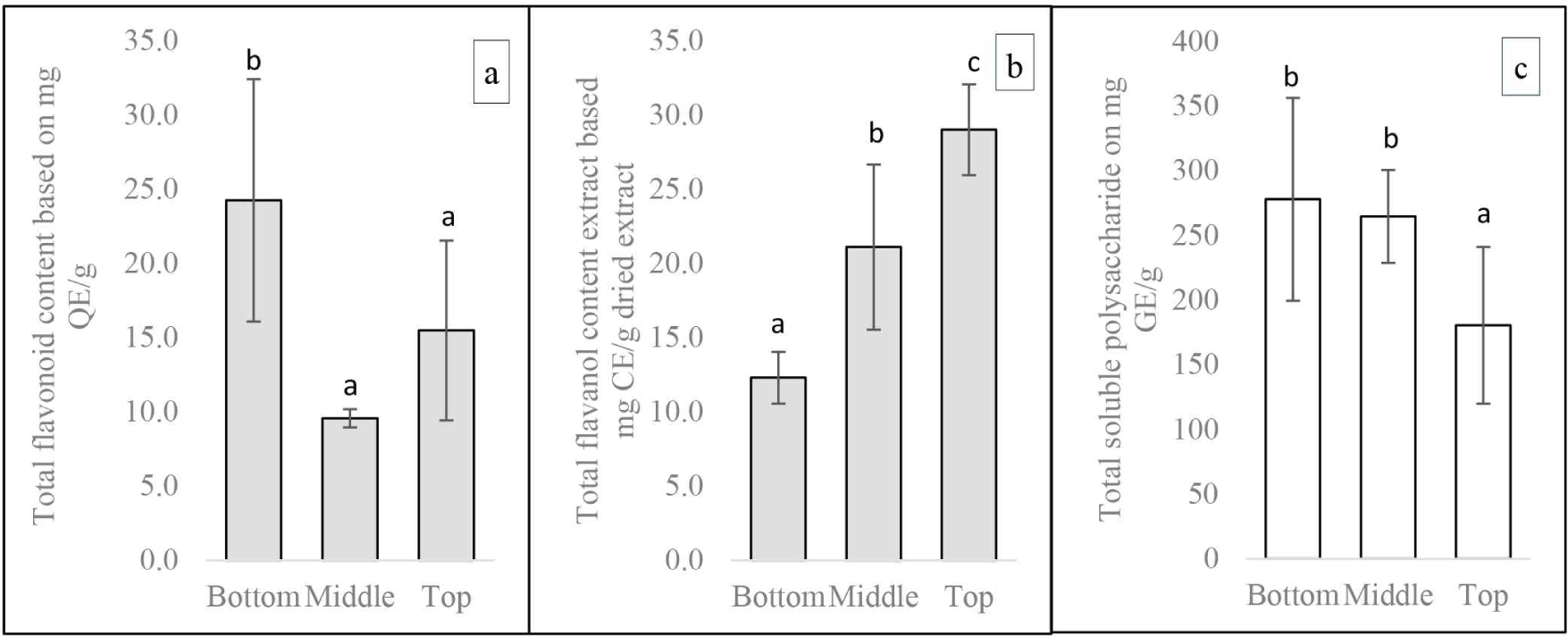
TSP levels were slightly higher near the pith than near the bark at the base parts. The reverse pattern was found in the middle and upper part, where TSP levels were slightly higher near the bark than near the pith (Table 3). This might be due to the influence of heartwood proportion. The content of TSP levels was lower near the pith than near the bark due to the base, which tends to have a higher proportion of heartwood. This might be because sugar components such as glucose, fructose, and sucrose are metabolized near the pith during heartwood formation to form phenolic compounds generally concentrated in the heartwood. Cui et al. (2020) reported that heartwood substances are formed through secondary metabolism in trees, with non-structural carbohydrates (NSC) acting as the primary metabolic substrates. Hillis (1987) stated that in trees, NSC serves as the primary photosynthetic storage compound, transported inward via ray parenchyma cells as secondary components of heartwood are formed. The main components of NSC are starch and soluble sugars, including sucrose, arabinose, glucose, fructose, stachyose, galactose (Cui et al., 2020).
The relationship between extractive and phenolic levels was presented in Table 5. Pearson’s correlation test was conducted only on differences in axial position, as the previous ANOVA test revealed more significant differences in axial position than radial position. At the base of the tree, extractive levels were generally positively correlated with TFC and TVC, except for the correlation between extractive levels and TPC, which was negatively correlated with both methanol extract (–0.82) and hot water extract (–0.09). Notably, there was a strong correlation between TPC and TFC of hot water extract (0.90*). Furthermore, a positive correlation was found between extractive levels and phenolics, particularly a significant correlation between methanol extract and TVC (0.85*), whereas the opposite pattern was observed in hot water extract (–0.24). At the top of the tree, a negative correlation was found between methanol extractive levels, TFC and TVC. Conversely, a positive relationship was detected between hot water extract and phenolic levels. Additionally, significant positive correlations were obtained between TFC and TVC of methanol extract (0.96*) and between TPC and TVC (0.91*).
A positive correlation suggests the presence of a specific compound type within the phenolic group, whereas a negative correlation implies the presence of a different or less prominent compound type. In this study, numerous positive correlations were found along the axial position between extractive levels (methanol and hot water) and TFC and TVC, indicating the presence of dominant flavanol compounds from the flavonoid group. Some of these flavonoid compounds may have been reported in previous studies in several Acacia species (Barry et al., 2005; Mihara et al., 2005; Seigler, 2003).
The IC50 levels ranged from 166.3 to 509.3 μg/mL, slightly lower than previous studies on Acacia hybrid clones 16, 25, and 44 which ranged from 255.7 to 680.5 μg/mL (Purba et al., 2021). This shows that the Purwo Sri Ah015D clone, especially the base part (Fig. 6) has slightly higher antioxidant activity than the previous three clones of Acacia hybrid. Furthermore, the results of the two-way ANOVA test showed that only the axial position had a significant effect (Table 4). The IC50 values at the bottom (255.8 ± 99.7 μg/mL) were significantly higher than those at the middle (438.0 ± 50.7 μg/ mL) and top (506.7 ± 35.0 μg/mL; Fig. 6). This trend was similar to the TPC and TFC patterns. This also might be due to the influence of the proportion of heartwood. Heartwood is more dominant at the base than at the top parts. Furthermore, the IC50 values in the bottom parts were around 9 and 10 times for gallic acid and quercetin, respectively.
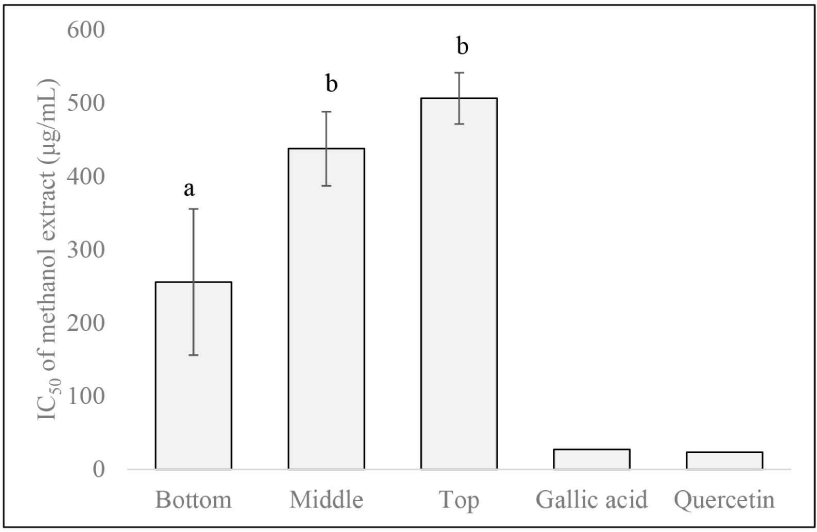
Purba et al. (2021) reported in Acacia hybrid clones (6 years) 16, 25, 44 that the IC50 value of heartwood were around 7.43 (outer) and 6.56 (inner) times of gallic acid and 6.37 (outer) and 5.62 (inner) times of catechin. In addition, Mihara et al. (2005) reported that activity of antioxidant in A. mangium and A. auriculiformis associated with antifungal activity and inhibition of laccase enzymes in the extract. They stated that the IC50 value was 14.53 times that of the gallic acid control in A. mangium extract, while A. auriculiformis has an IC50 value of 3.76 times. Phenolic compounds are also thought to have the ability to clean up hydroxyl radicals that are created by the laccase enzymes of the heart decay fungi.
Given the known role of phenolic compounds in fungal resistance, the observed high TPC and low IC50 values suggest a potential correlation with improved heart rot resistance. Pearson correlation analysis was used to investigate the correlation between extractives, phenolics, flavonoids, and flavanols with antioxidant activity, measured by radical scavenging ability, across different axial positions (Table 6). Flavonoid groups showed a good negative correlation with antioxidant activity at the base (–0.69) and in the middle (–0.48) parts, suggesting that TFC and TVC are key drivers of antioxidant activity in these regions, where heartwood is more abundant [Fig. 7(a) and (b)]. The same pattern was also observed in the correlation between extractive levels and IC50 (Table 6). This finding was supported by the Pearson correlation results in Table 5. Conversely, at the top, antioxidant activity was positively correlated with the phenolic group, suggesting that other compounds, such as sugars and fats typically found in sapwood, may play a more significant role in this region.
| Antioxidant | Pearson correlation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bottom | Middle | Top | ||||||||||
| Extractive | TPC | TFC | TVC | Extractive | TPC | TFC | TVC | Extractive | TPC | TFC | TVC | |
| IC50 | 0.27 | –0.36 | –0.69 | 0.10 | –0.07 | 0.08 | –0.21 | –0.47 | –0.60 | 0.48 | 0.52 | 0.60 |
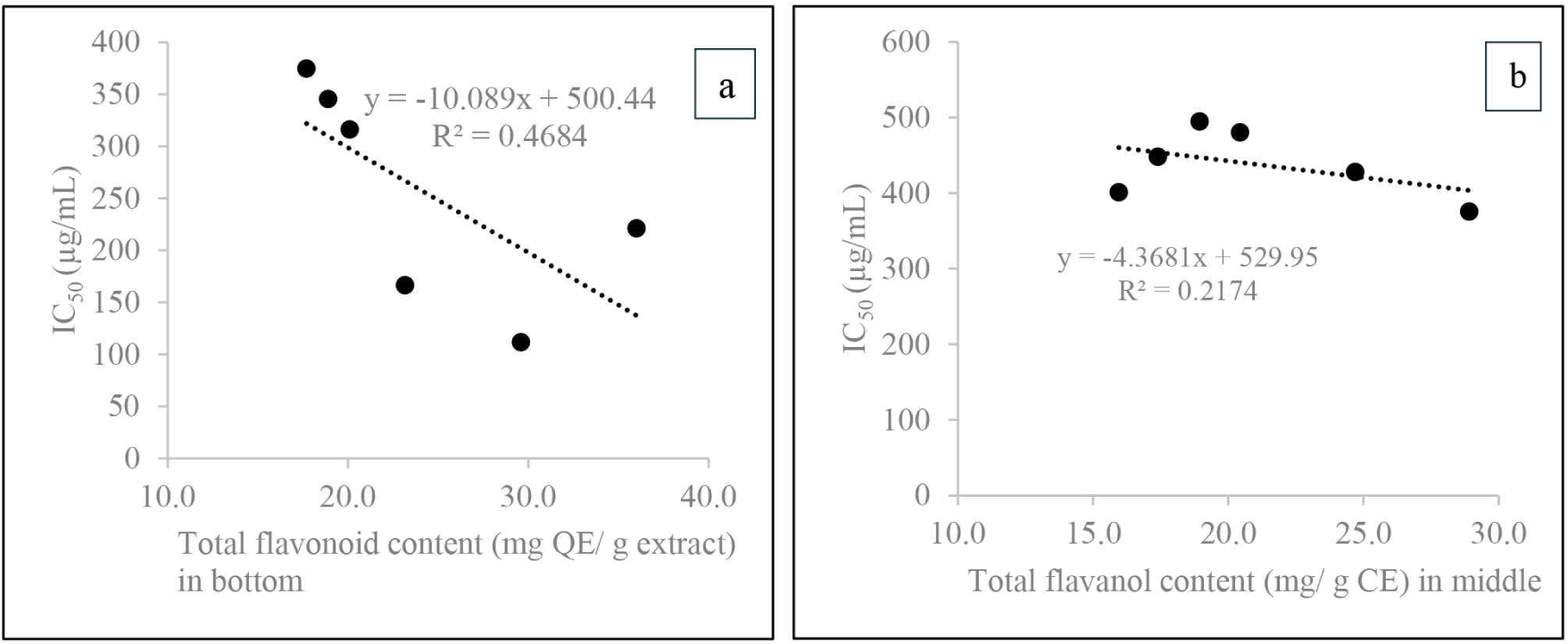
The Pearson correlation analysis results highlight the dominant role of flavonoid compounds in influencing antioxidant activity. Moreover, certain flavonoid compounds not only act as antioxidants but also contribute to natural resistance against heart root disease, which commonly affects several Acacia species. Previous studies found three flavonoid dominant compounds, namely 2,3 trans-3,4’,7,8-tetrahydroxyflavanone, teracacidin, and 4’,7,8,-trihydroxyflavanone in A. mangium and A. auriculiformis with antifungal activity (Barry et al., 2005; Mihara et al., 2005; Pietarinen et al., 2004). The study only covered the general composition of TPC, TFC, TVC, TSP, and IC50. Therefore, further studies are needed to identify the specific flavonoid compounds that are dominant in the Acacia hybrid clone Ah015D and determine which compounds are responsible for its natural resistance. Additionally, a methanol-water solvent combination can be employed to discover compounds that are poorly soluble in methanol or hot water, potentially revealing new insights.
4. CONCLUSIONS
In conclusion, the mean contents of n-hexane, methanol, as well as hot water extractive of wood from Acacia hybrid Variety Purwo Sri Ah015D ranged from 0.36% to 0.73%, 0.94% to 1.63% and 0.58% to 0.82%, respectively. The content of n-hexane extract was significantly influenced by the interaction of radial and axial positions. In contrast, methanol and hot water extractive contents were not significantly affected by radial or axial position. TPC, TFC, TVC, and TSP in methanol extract ranged from 135.7 to 402.9 mg GAE/g, 9.55 to 29.6 mg QE/g, 12.2 to 31.7 mg CE/g, 368.5 to 475.4 mg GE/g, respectively. For the hot water extract, ranged from 50.3 to 81.0 mg GAE/g, 5.81 to 7.59 mg QE/g, 42.9 to 64.0 mg CE/g, and 139.2 to 330.5 mg GE/g dried extract. Furthermore, the values of IC50 ranged from 166.3 to 509.3 μg/mL, with the highest value at the bottom (255.8 ± 99.7 μg/mL) and the lowest at the top part (506.7 ± 35.0 μg/mL). Two-way ANOVA analysis showed that the interaction between radial and axial positions significantly influenced the value of TPC. Meanwhile, the concentrations of TFC, TVC, TSP and IC50 were significantly affected by the difference in axial position. The low concentration of non-polar extractives, which are generally lipophilic compounds, and the low heartwood proportion suggest that Acacia hybrid Variety Purwo Sri Ah015D is suitable for utilization as a pulp raw material. In addition, Pearson correlation analysis showed a positive relationship between the levels of methanol and hot water extractives on TFC and TVC values along the axial position. This indicates the dominance of certain flavonoid compounds that have the potential as natural durability against heart rot and have high antioxidants. This pattern was also supported by the results of the Pearson correlation analysis between flavonoid groups and IC50 which showed a negative correlation. Therefore, further studies are needed to identify certain flavonoid compounds that are responsible for natural resistance and antioxidant activity in Acacia hybrid. Furthermore, these compounds may also have the potential as a source of natural medicine.