1. INTRODUCTION
Palladium (Pd) is an exceptionally rare element, found only in limited regions of the world—such as South Africa, Russia, and Ontario, Canada—where it occurs at concentrations as low as 0.1 to 3 ng/g in the crust of the Earth (Liu et al., 2024). Extensive industrial Pd use has led to an increase in the generation of Pd-containing wastewater. Without appropriate treatment, this waste poses a significant risk of environmental contamination. Therefore, the recovery of Pd from industrial effluents is vital for reclaiming this valuable resource and mitigating environmental pollution.
In parallel with these environmental concerns, Pd is also a critical platinum-group metal (PGM) with a high industrial demand, particularly for semiconductor inspection equipment, automotive catalytic converters, petroleum-refining catalysts, and fuel cell vehicles (Das and Linert, 2016; Ohmatsu et al., 2012; Yamada et al., 2015). For example, Pd alloy materials are primarily used in pogo pins for test sockets during semiconductor production inspection processes (Tseng et al., 2024). Various processing techniques have been employed in plunger manufacturing to achieve a low electrical resistivity, high flexibility, and broad range of hardness values. During the manufacturing process, a significant amount of Pd alloy scrap is generated as a byproduct, and this is typically classified as melting scrap and processing scrap (Yamada et al., 2023). Therefore, recovering Pd from the by-product scrap generated in the plunger manufacturing process for pogo pins presents a valuable opportunity (Hammadi et al., 2017).
Conventional Pd recovery methods include hydrometallurgy (Quinet et al., 2005), ion exchange (Nikoloski et al., 2015), solvent extraction (Paiva, 2017), and chemical precipitation (Yousif, 2019). However, these methods have disadvantages, such as high chemical consumption, secondary contamination, and low selectivity for Pd recovery. In contrast, adsorption offers advantages such as process simplicity, sustainability through repeated adsorption and desorption, and minimal secondary byproduct generation (Veglio and Beolchini, 1997; Wang et al., 2015).
Lignin, a major component of green biomass, is the second most abundant natural polymer after cellulose (Bang et al., 2022). Owing to its complex chemical network, lignin has traditionally been utilized as an energy source (Park et al., 2020). Recently, its applicability has expanded to include use as a carbon material precursor (Lee et al., 2021), emulsifier (Gao et al., 2022), functional additive in cosmetics (Antunes et al., 2023), adhesive filler (Yang et al., 2019), UV-blocking film (Zheng et al., 2023), food packaging material (Zubair et al., 2024), and bio-based polyol for polyurethane production (Won et al., 2024). In addition, lignin has been extensively investigated as an adsorbent material because of its diverse functional groups, including phenolic, aliphatic, and carboxyl groups (Kim et al., 2023). However, the composition of lignin monomers, such as sinapyl alcohol (S), p-coumaryl alcohol (H), and coniferyl alcohol (G), varies significantly depending on the tree species and growth conditions (Park et al., 2018). Notably, the methoxy groups adjacent to the phenolic hydroxyl groups contribute to cellulose fiber bonding but also hinder adsorption interactions between lignin and contaminants (Yunus et al., 2024).
In this study, we investigated the effects of methoxy group substitution with hydroxyl groups on Pd adsorption after lignin demethylation. Structural modifications induced by demethylation were analyzed via Fourier-transform infrared (FTIR) and phosphorus-31 nuclear magnetic resonance (31P NMR) spectroscopy. Furthermore, adsorption experiments were conducted to evaluate the effect of increasing the hydroxyl content on Pd–lignin interactions. Based on these findings, enhancing lignin hydroxyl functionality, via demethylation, could be proposed as a fundamental strategy for improving adsorption efficiency, not only for Pd recovery, but also for the removal of various waterborne contaminants.
2. MATERIALS and METHODS
Hardwood kraft lignin (KL), lithium bromide (LiBr, 99%), hydrobromic acid (HBr, Extra pure, 47% to 49%), palladium (II) chloride (PdCl2, 99%), hydrochloric acid (HCl, ACS grade, 36.5% to 38.0%), and sodium hydroxide (NaOH, Extra pure), pH 2 buffer solution, were used. All chemicals were used without further purification, and distilled water was used throughout the study.
Demethylated kraft lignin (DKL) was prepared using an acidic concentrated lithium bromide (ACLB) system, and the 61% LiBr aqueous solution was prepared by mixing 24.4 g of LiBr and 15.6 g with distilled water. Moreover, 1 g of KL was added to a 61% LiBr aqueous solution and stirred, and then, 4 mL of 48% HBr was added. The solution was allowed to react in a small reactor in an oil bath at 120°C for 30 min. After the reaction, filtration and washing were performed with distilled water, and the solution was dried in an oven at 60°C to obtain the DKL.
PdCl2 (50 mg) was added to a solution of 4 mL of 36% HCl and 16 mL of distilled water and completely dissolved. Additionally, 80 mL of distilled water was added to this solution to prepare a 500 ppm PdCl2 aqueous solution. The pH was set to 2 using a pH meter and an aqueous NaOH solution. Using a pH 2 buffer solution, Pd aqueous solutions with concentrations of 200 ppm (the concentration of Pd, not the concentration of PdCl2) were prepared.
The chemical structures of KL and DKL were analyzed through FTIR (Summit, Thermo Fisher Scientific, Waltham, MA, USA) and 31P NMR (Advance 600 MHz high-resolution spectrometers, Bruker, Berlin, Germany). FTIR analysis was performed in Attenuated Total Internal Reflection mode, and the range of 4,000–525 cm–1 was measured at a 4 cm–1 resolution with 64 scans. 31P NMR analysis was performed to analyze the distribution and content of hydroxyl groups in the lignin. To this end, 40 mg of KL and DKL was dissolved in 0.5 mL of a mixture of anhydrous pyridine and chloroform-d (Deuterated Chloroform, CDCl3), and 0.2 mL of a standard reagent (e-NHI, N-hydroxy-5norborene-2, 3-diimide) was added. The sample was prepared by mixing with 0.05 mL of a stabilizer [chromium (III) acetylacetonate solution] and then phosphate treatment with 0.1 mL of a phosphoric acid chloride reagent (2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, TMDP).
Gel permeation chromatography (GPC, LC-40, Shimadzu, Kyoto, Japan) was performed to measure the molecular weight of the lignin. Each sample was dissolved in tetrahydrofuran at 1 mg/mL, filtered through a 0.45 μm polytetrafluoroethylene syringe filter (Advantech, Tokyo, Japan), and analyzed at a flow rate of 1 mL/min. GPC calibration was performed using ReadyCal Kit Poly (styrene) low (PSS Polymer Standards Service, Mainz, Germany), and the number-average molecular weight (Mn), weight-average molecular weight (Mw), and polydispersity index (PDI) of the lignin samples were calculated.
The surface charges of the lignin samples were investigated using a Zetasizer (Litesizer 500, Malvern, UK), depending on the pH of the solution. To measure water solubility, 1 g of each lignin sample was immersed in 30 mL of aqueous solutions of pH 2, 7, and 10 and stirred at 25°C for 1 day. Subsequently, the undissolved samples were separated via centrifugation for 10 min at 6,800×g. Undissolved samples were dried in an oven at 60°C for 12 h.
First, 10 mg of the lignin sample was added to 10 mL of a 200 ppm Pd aqueous solution, and adsorption experiments were performed at 25°C for 30 min with stirring at 180 rpm. The concentration of Pd before and after all adsorption experiments was measured via inductively coupled plasma-atomic emission spectroscopy (ICP-AES, ICP5800, Agilent, Santa Clara, CA, USA). The Pd adsorption capacity of the lignin was calculated using the following Equation (1):
Where C0 and Ce are the initial and equilibrium concentrations of the aqueous Pd solution (mg/L), respectively. V denotes the volume (l) of the Pd aqueous solution and M is the mass (g) of the lignin-based adsorbent. The atomic elements and chemical functional groups on the surfaces of the lignin samples before and after Pd adsorption were analyzed using X-ray photoelectron spectroscopy (XPS, AXIS SUPRA, Kratos, Manchester, UK).
3. RESULTS and DISCUSSION
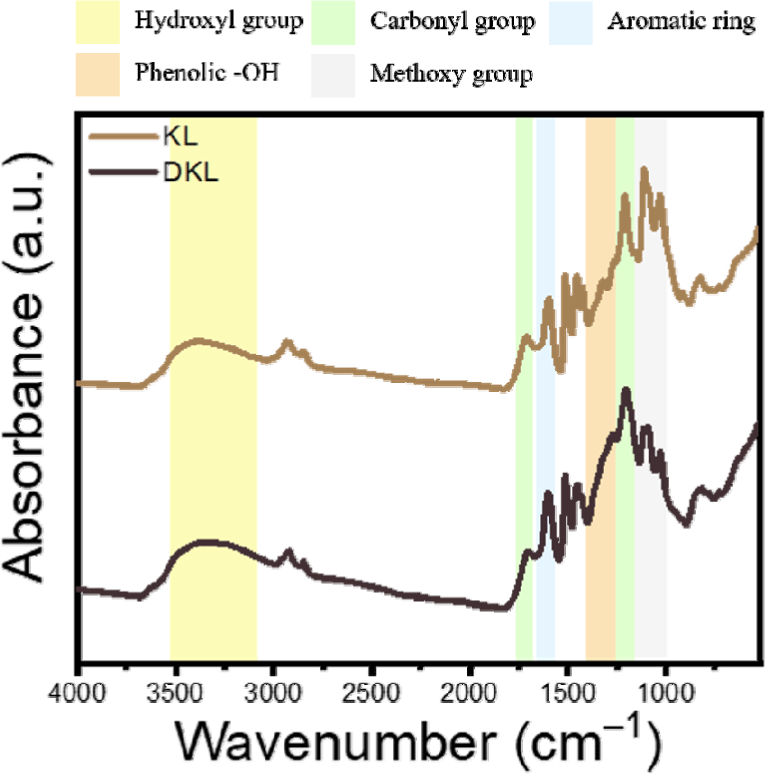
Based on the FTIR analysis results for KL and DKL, it was predicted that the phenolic hydroxyl groups of lignin were increased through demethylation (Fig. 1). A peak at 1,710 cm–1, corresponding to the carbonyl group, was observed for KL and DKL (Hwang et al., 2021). In the case of KL, it showed distinct peaks at 3,500–3,200 cm–1 attributed to hydroxyl groups and 1,600 cm–1 due to the C = C bond of the aromatic ring (Watumlawar, 2023). In addition, it showed characteristic peaks at 1,120 cm–1 and 1,030 cm–1 owing to the C-H bond of the methoxy group (Lee et al., 2019). However, for DKL that underwent demethylation, a significant decrease in the intensities of the peaks at 1,120 and 1,030 cm–1 was observed, which showed a decrease in the methoxy structure (Demuner et al., 2024; Fiţigău et al., 2013). In addition, compared to that with KL, the intensity of the peak at 3,500–3,200 cm–1 corresponding to the hydroxyl groups increased with DKL, confirming that the formation of hydroxyl groups increased through the demethylation process. Additionally, the FTIR peak at approximately 1,375 cm–1 for DKL indicates an increase in phenolic OH compared to that with KL (Pandey, 1999).
The hydroxyl content of the lignin before and after demethylation was calculated using 31P NMR spectroscopy, and the results are presented in Fig. 2 and Table 1. KL and DKL showed chemical shifts and intensity changes in both the phenolic and aliphatic OH regions in the 31P NMR spectra. For aromatic hydroxyl groups, the rapid increase in the aromatic hydroxyl content in DKL, from 2.43 mmol/g to 7.11 mmol/g, was attributed to demethylation of the methoxy groups in the ACLB system via SN2 nucleophilic substitution, in which bromide ions cleave the ether bonds (Li et al., 2020). Moreover, methyl-O-aryl ether linkages, such as α-O-4, β-O-4, and α-O-γ, were also cleaved in the ACLB system, leading to the formation of new phenolic hydroxyl groups (Li et al., 2018). However, in the case of aliphatic hydroxyl groups, this decreased from 0.98 mmol/g to 0.31 mmol/g. The loss of aliphatic hydroxyl groups presumably resulted from the acid-catalyzed elimination of the aliphatic hydroxyl groups, and the primary hydroxy group was brominated in the ACLB system (Tao et al., 2016; Wang et al., 2023).
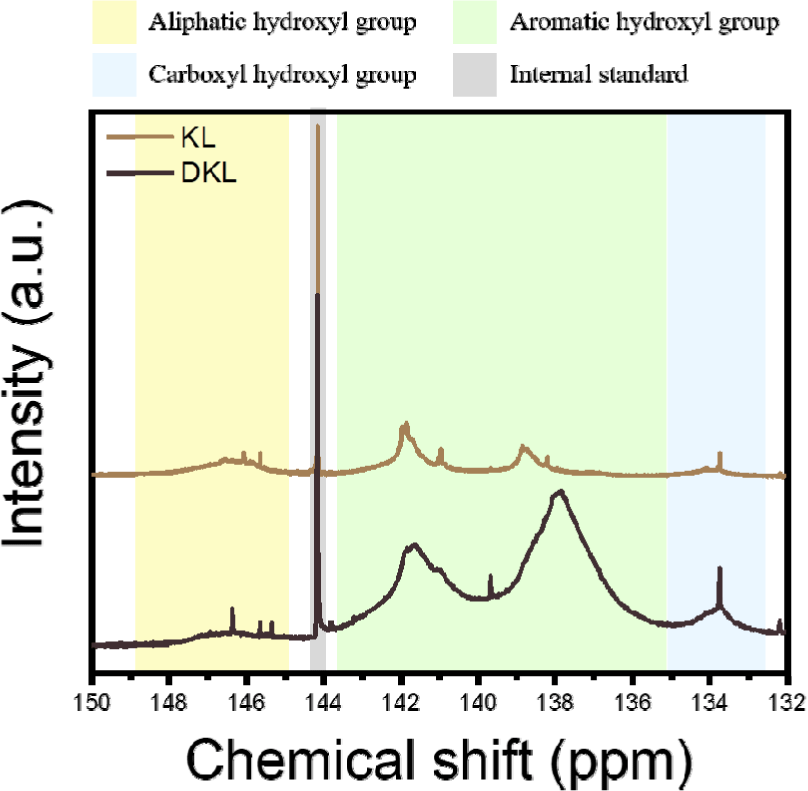
| Aliphatic hydroxyl group (mmol/g) | Aromatic hydroxyl group (mmol/g) | Carboxyl hydroxyl group (mmol/g) | Total (mmol/g) | |
|---|---|---|---|---|
| KL | 0.98 | 2.43 | 0.18 | 3.58 |
| DKL | 0.31 | 7.11 | 0.83 | 8.25 |
In general, the stability of lignin in an aqueous environment is vulnerable to alkaline conditions and is greatly affected by its molecular weight and hydroxyl group content (Evstigneev, 2011). GPC analysis was performed to observe the change in the molecular weight of lignin due to the demethylation process, and the results are shown in Fig. 3. Fig. 3(a) and (b) present the GPC results for KL and DKL, showing the molecular weight, PDI, and molecular weight distribution. For DKL, a peak was observed at a shorter retention time than that for KL, indicating that condensation occurred during demethylation [Fig. 3(c)]. As shown in Table 2, the Mn values for KL and DKL were 649 and 947 Da, respectively, the Mw values were 2,116 and 5,784 Da, respectively, and the PDI values were 3.26 and 6.11, respectively, indicating that condensation occurred in DKL. Li et al. (2020) reported that the demethylation of lignin is carried out in ACLB systems and that elevated temperatures promote lignin repolymerization through acid-induced condensation reactions, such as those between aromatic C5 or C6 positions and benzyl cations (Li et al., 2020). This increase in the molecular weight due to condensation during demethylation also greatly affects the solubility of DKL. As shown in Fig. 3(d), KL exhibited solubilities of 18% and 29% at pH 2 and 7, respectively, which means that lignin was eluted when used as a water treatment material. Meanwhile, in the case of demethylated DKL, the stability in the aqueous environment increased owing to the effect of an increasing molecular weight, despite the increase in the hydroxyl group content. As a result, it showed a low solubility of less than 3% under both pH 2 and 7 conditions. This indicates that it can be used as a sustainable adsorption material under both acidic and neutral conditions for water treatment purposes.
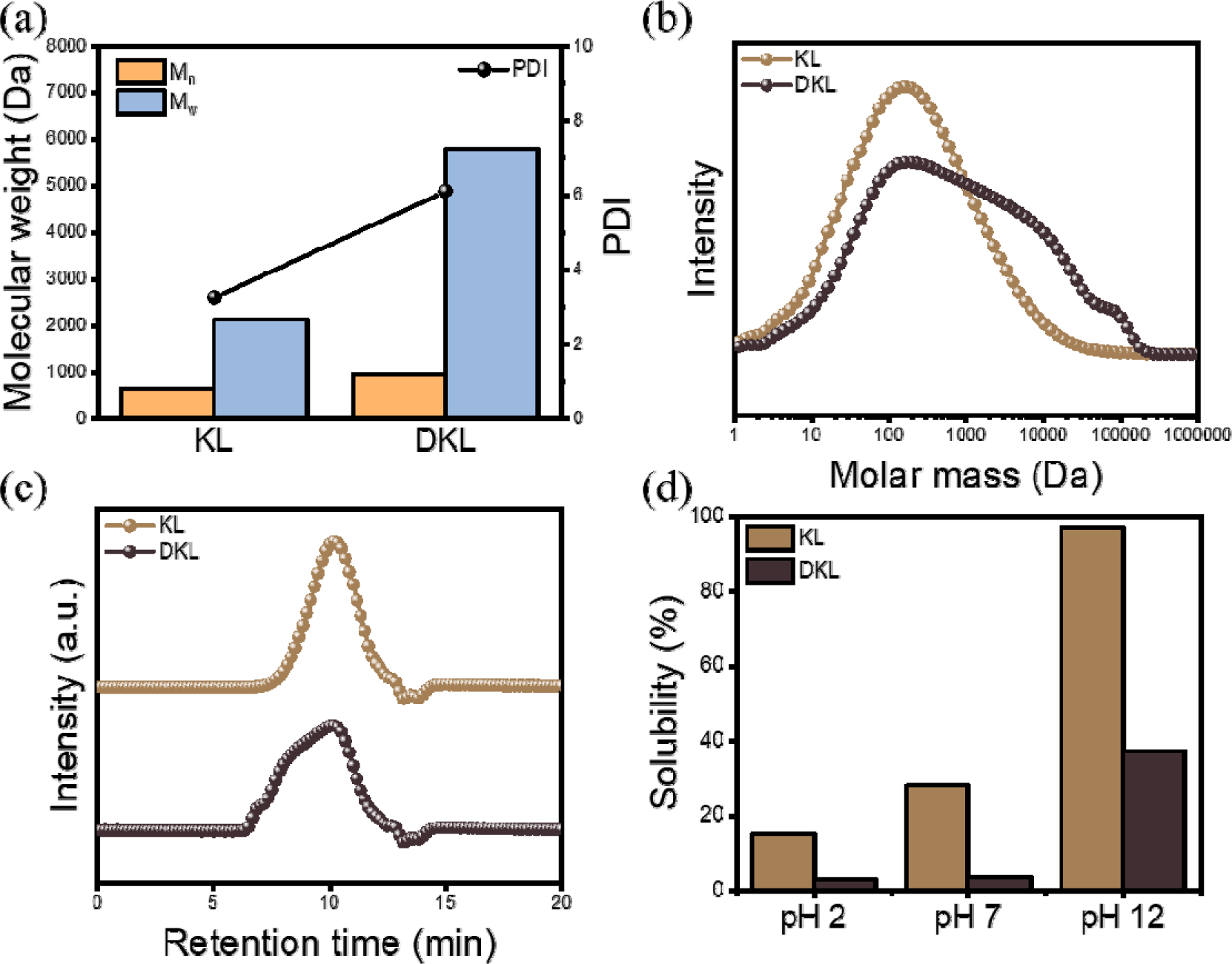
| Mn (Da) | Mw (Da) | PDI | |
|---|---|---|---|
| KL | 649 | 2,116 | 3.26 |
| DKL | 947 | 5,784 | 6.11 |
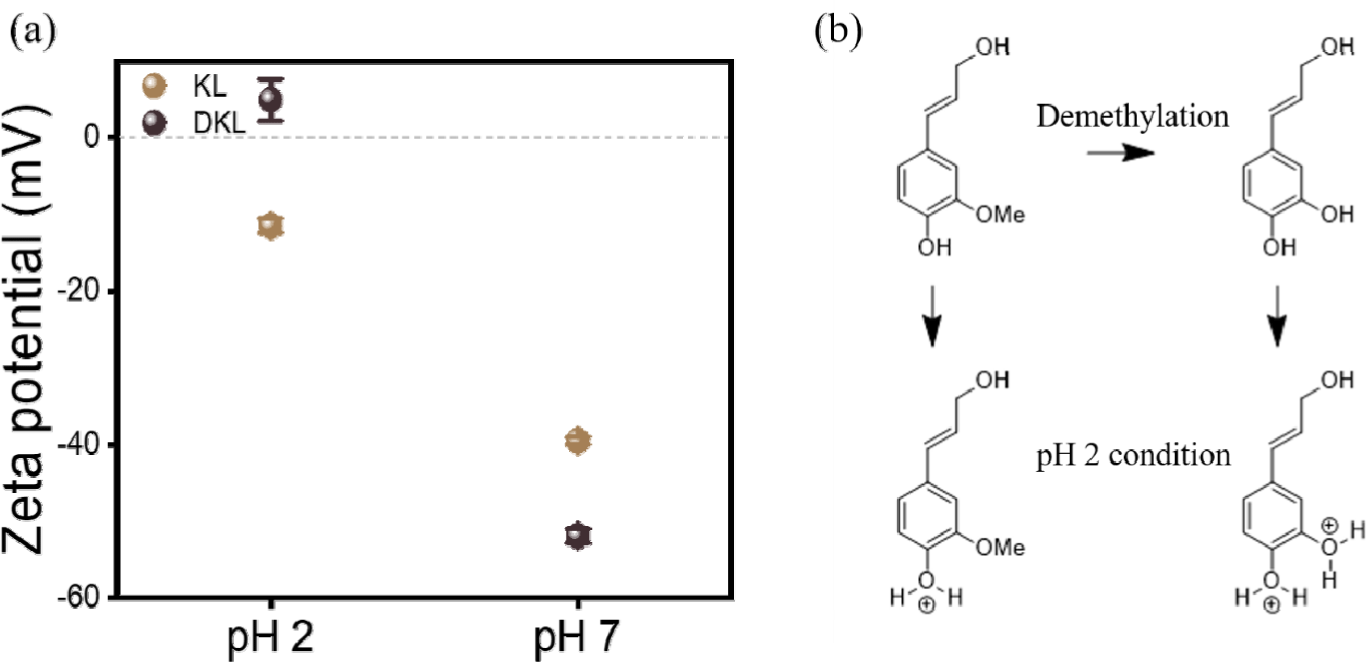
When utilizing lignin as a heavy metal adsorption material, not only the content of hydroxyl groups but also the structural stability in various pH environments are important characteristics (Kim et al., 2024). To examine the changes in surface charge characteristics resulting from lignin demethylation, the zeta potential was measured, and the results are shown in Fig. 4(a). For KL, negative charges of –18.2 mV at an acidic pH of 2 and –41.5 mV under pH 7 conditions were detected. This negative charge characteristic is because the hydroxyl and carbonyl groups present in lignin are the sites that mainly show charge characteristics (Chipman, 1986; Wiberg, 1999). However, for DKL, with an increased phenolic hydroxyl group content, a value of +5.3 mV was detected at pH 2, indicating that it can show positive charge characteristics under acidic conditions (Zhu et al., 2024). Meanwhile, a value of –56.4 mV was detected at pH 7, which occurred because demethylation introduces more hydroxyl groups into the lignin molecular backbone, causing strong deprotonation and protonation [Fig. 4(b)]. Therefore, since DKL can have a positive surface charge under acidic conditions, such as at pH 2, unlike KL, it is expected that the electrostatic attraction-based adsorption of negatively charged contaminants will be possible.
In general, in the metal industry, including pogo pins for semiconductor inspection, Pd scrubs can be obtained by leaching with strong acids, such as sulfuric acid and hydrochloric acid, to obtain waste liquid. Therefore, a model Pd solution was prepared by dissolving PdCl2 in a solution of hydrochloric acid at pH 2. As shown in Fig. 5(a), the PdCl2 solution had an intense yellow color when prepared at 200 ppm, which is a phenomenon that occurs when Pd compounds are dissolved. KL and DKL were added to the Pd solution for the adsorption experiments. When KL was added, the color reduction of the Pd solution before and after adsorption was not significant, indicating that considerable Pd adsorption was not evident, even to the naked eye. However, when DKL was added, the yellow color of the Pd solution after adsorption was lighter and more transparent than that before adsorption, which indirectly indicates that significant adsorption had occurred.
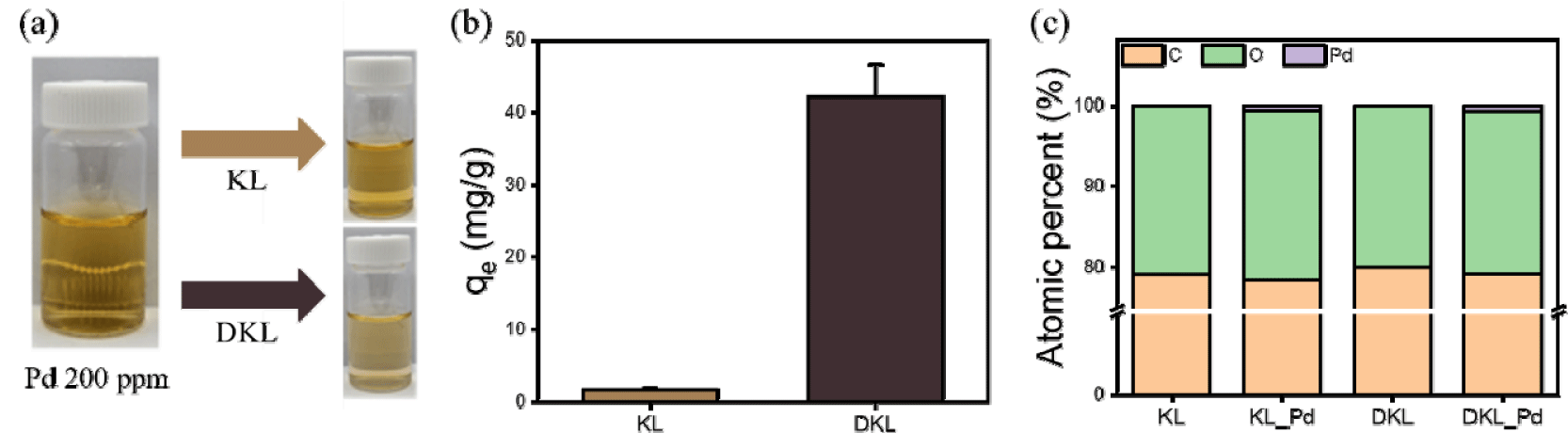
To examine the actual Pd adsorption capacity, the Pd concentrations before and after adsorption were analyzed using ICP-AES [Fig. 5(b)]. The calculated Pd adsorption capacity of DKL was significantly higher than that of KL, at 42.2 mg/g compared to 4.5 mg/g, respectively. This excellent Pd adsorption capacity was also consistent with the color fading, visible to the naked eye, after adsorption. This phenomenon is attributed to the surface positive charge tendency of DKL under acidic conditions, which is caused by an increase in the phenolic hydroxyl groups in the demethylation process, strengthening the electrostatic attraction with PdCl42– and enabling the recovery of a larger amount of the Pd compound (Zhu et al., 2024). The Pd contents of KL and DKL, which were confirmed through elemental composition analysis obtained from the actual XPS full spectrum [Fig. 5(c)], also showed a higher Pd content in DKL, indicating that Pd was adsorbed on the surface of DKL.
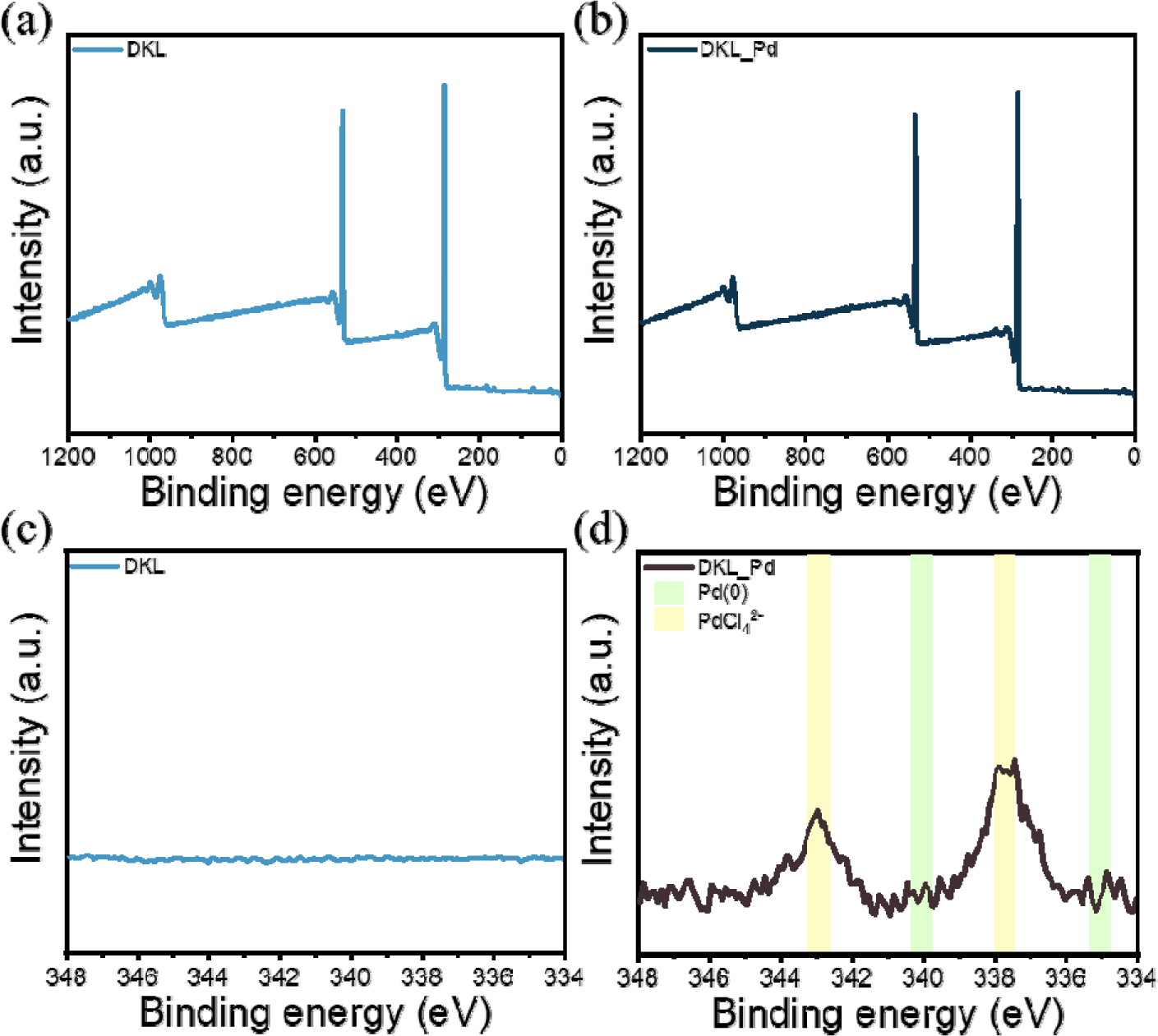
To understand the Pd adsorption behavior of DKL, XPS was performed on the adsorbent surface before and after adsorption, and the high-resolution XPS spectrum in the Pd detection region of 345–335 eV is shown in Fig. 6. For DKL, before adsorption, no noticeable Pd peak was detected in the range of 335–345 eV [Fig. 6(a) and (c)]. However, after adsorption, a distinct Pd 3d peak appeared, indicating that the Pd compounds were successfully adsorbed onto the DKL surface [Fig. 6(b) and (d)]. In particular, as shown in Fig. 6(d), the Pd 3d5/2 and Pd 3d3/2 XPS spectra showed strong intensities at 343.0 eV and 337.8 eV, respectively; these are typical characteristics of Pd(II) and are consistent with the results when it exists as PdCl42– (Savastano et al., 2019). In addition, the fact that the characteristic peaks of Pd(0) at 345.0 eV and 340.5 eV were not detected indicates that the reduction of Pd adsorbed on the surface of DKL did not occur (Karhu et al., 2003). This indicates that electrostatic interactions between the surface of DKL and PdCl42– could be the main adsorption mechanism (Zhu et al., 2024). Kim et al. (2024) reported that in the case of lignin modified via cationization using polyethyleneimine, the surface was enriched with amine groups, which increased the electrostatic interaction with the anionic Pd compound (Kim et al., 2024).
4. CONCLUSIONS
In this study, we investigated the possibility of using lignin as an eco-friendly adsorbent to recover Pd from Pd-scrub wastewater generated at industrial sites. For this purpose, the demethylation of KL was performed under ACLB conditions. For the obtained DKL, the content of aromatic hydroxyl groups increased by approximately 190%, and at the same time, the molecular weight increased owing to repolymerization, which resulted in excellent stability in an aqueous environment. Unlike KL, DKL exhibited positive charge characteristics under acidic conditions (pH 2), which enabled electrostatic interactions with PdCl42–. DKL showed excellent Pd adsorption capacity (42.2 mg/g) compared to KL (4.5 mg/g). In addition, it is expected that the Pd recovery efficiency can be maximized through additional chemical modification utilizing the increased phenolic hydroxyl groups of lignin as the main functional group. These results suggest that lignin can be utilized as a useful biomass-based adsorbent for the recovery of PGMs, including Pd, and that it also has the potential to be upcycled into a high-performance catalyst by reducing the amount of Pd adsorbed on the surface of lignin.








