1. INTRODUCTION
Ulin wood (Eusideroxylon zwageri Teijsm. & Binn.), commonly referred to as ironwood, is a tropical hardwood native to Kalimantan, Indonesia. Known for its exceptional durability and density, it is classified as a Class I strong and durable wood with outstanding resistance to decay, mechanical stress, and termite attacks (Savero et al., 2020; Wong et al., 2012). These properties have proven important in various applications such as construction, shipbuilding, and the production of high-quality furniture (Abdurachman et al., 2022; Irawan, 2016). However, the widespread use of ironwood, combined with its slow growth and difficult breeding process, has led to its inclusion in the International Union for Conservation of Nature Red List as a vulnerable species (Asian Regional Workshop, 1998). This highlights the urgent need for sustainable utilization and conservation strategies.
The durability of ironwood is due to its high levels of extractive compounds, which vary based on factors like wood type, density, tree age, environmental conditions, and geographic origin (Kirker et al., 2024; Oh et al., 2023). These extractives, especially secondary metabolites like polyphenols, flavonoids, and other phenolic compounds, are recognized for their bioactive properties, including antioxidant, antimicrobial, antifungal, and anti-termite activities (Chaerunisaa et al., 2020; Sankara et al., 2020). Among these, polyphenols are key in boosting wood resistance by scavenging free radicals, preventing oxidative degradation, and showing toxic effects against wood-destroying organisms such as termites (Kusuma et al., 2018; Nkogo et al., 2022).
Termites, particularly Cryptotermes cynocephalus, are among the most destructive pests of dry wood, causing significant economic loss and ecological damage worldwide (Romano and Acda, 2017). Traditional termite control methods usually depend on synthetic chemicals that can harm the environment and health. This has sparked growing interest in plant-derived natural termiticides as sustainable alternatives (Adfa et al., 2023; Oi, 2022). Ironwood, with its rich extractive composition, has shown promise as a source of bioactive compounds with anti-termite potential. Phenolic derivatives such as eusiderin, condensed tannins, and lignin have been identified as potentially toxic to termites (Timotius and Rahayu, 2021).
Despite its well-known durability and environmental importance, detailed studies on the bioactive properties of ironwood are still limited. This study aimed to address this gap by exploring the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activities of ethanol extracts from 15 accessions of ironwood collected from Kalimantan, Indonesia. The antioxidant potential was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric-reducing antioxidant power (FRAP) assays, while anti-termite activity was evaluated against C. cynocephalus using termite mortality, paper weight loss, and antifeedant tests. By identifying and characterizing the bioactive compounds responsible for antioxidant and anti-termite activities, this study aims to offer an environmentally sustainable approach to wood preservation and promote the conservation and sustainable use of ironwood resources. This research also represents a first step toward understanding the genetic basis of wood quality and termite resistance, which could potentially be applied to modify other non-endangered woods to develop high-quality wood without exploiting ironwood.
2. MATERIALS and METHODS
The ironwood branches were collected from the National Research and Innovation Agency plant collection at the Bogor Botanical Garden, Indonesia, between March and April 2023. In this study, 15 ironwood accessions originating from various regions of Kalimantan of different tree ages were used (Table 1). The collected wood branch samples were carefully washed under running water to remove impurities and subsequently oven-dried at 40°C for 72 h. The dried branches were cut into smaller pieces to facilitate grinding. The resulting pieces were processed using a grinding machine to obtain a fine powder with a particle size of 60 mesh.
Data from Ariati et al. (2019).
The extraction process was carried out by the maceration method using 96% ethanol as the solvent. A total of 30 g of ironwood branch powder from each accession was dissolved with 150 mL of 96% ethanol (1:5 w/v) in a 250 mL Erlenmeyer flask. The mixtures were then sonicated for 30 min in a sonicator set to 50–60 Hz (Decon F5 Major, Decon Laboratories, King of Prussia, PA, USA). After sonication, the mixtures were placed in a water bath shaker at 85 rpm for 24 h to complete the maceration process. The macerated solution was filtered using vacuum filtration with filter paper (Whatman No. 40, Whatman, Maidstone, UK). The obtained extracts were concentrated using a rotary vacuum evaporator at 50°C with a rotation speed of 40–70 rpm. The remaining solvent was further removed by drying the extract in an oven at 50°C until a completely dry powder was obtained. The extracts were kept in sealed glass bottles covered with aluminum foil and stored in a refrigerator for later analysis. For analysis, an ironwood extract solution was prepared by dissolving the dry extract in ethanol.
TPC was determined using the Folin-Ciocalteu method (Nofita et al., 2020). A 20 μL aliquot of the ironwood branch extract solution was added to a well of a 96-well microplate, followed by 120 μL of 10% Folin-Ciocalteu reagent. The mixture was then incubated for 5 min. Subsequently, 80 μL of 10% sodium carbonate (Na2CO3) was added, and the mixture was incubated at room temperature in the dark for 30 min. The absorbance was measured at 750 nm using a UV-Vis spectrophotometer. Gallic acid (Merck KGaA, Darmstadt, Germany) was used as the standard for with a calibration curve (y = 0.0042x + 0.0245, R2 = 0.9956) across a concentration range of 50–225 μg/mL. The TPC was calculated based on the gallic acid calibration curve and expressed as gallic acid equivalents (mg GAE/g dry weight) using formula (1):
Where, C = TPC (mg GAE/g DW); c = concentration of the extract (mg/mL); v = volume of the extract (mL); W = weight of the extract (g).
TFC was evaluated using the aluminum chloride (AlCl3) method, as described by Nofita et al. (2020). A 10 μL aliquot of the ironwood extract solution was added to a well of a 96-well microplate, followed by 60 μL of 96% ethanol. Subsequently, 10 μL of 10% AlCl3, 10 μL of potassium acetate (CH3COOK) 1 M, and 120 μL of distilled water were added sequentially. The mixture was incubated at room temperature in the dark for 30 min. Absorbance was measured using a UV-Vis spectrophotometer at a wavelength of 415 nm. Quercetin (Merck KGaA) was used as a reference with a calibration curve (y = 0.0013x – 0.0332, R2 = 0.9906) spanning 100–800 μg/mL. The TFC was calculated by referring to the quercetin calibration curve and expressed as quercetin equivalents (mg QE/g extract) using formula (1) described earlier.
Antioxidant activity was measured using the DPPH radical scavenging activity assay, as described by Rafi et al. (2019) with some modifications. The ironwood branch extract solutions were prepared at different concentrations (3.125, 6.25, 12.5, 25, 50, 100, 200 ppm). A 100 μL aliquot of each sample test solution was pipetted into a well of a 96-well microplate, followed by the addition of 100 μL of 50 μg/mL DPPH solution (w/v in ethanol). A blank solution was prepared by combining 100 μL of 96% ethanol and 100 μL of 50 μg/ mL DPPH solution. The mixtures were then incubated for 30 min at room temperature under dark conditions. The absorbance of DPPH was measured at 517 nm using a UV-Vis spectrophotometer. The same process was applied to Trolox (Merck KGaA), which served as the positive control. The inhibitory effect of DPPH was calculated using formula (2):
Antioxidant activity was expressed as the IC50 value (inhibitory concentration required to achieve 50% radical scavenging activity) and compared to the IC50 value of Trolox.
Antioxidant capacity was evaluated using the FRAP method, as described by Rafi et al. (2019). The FRAP reagent was prepared by mixing 10 mM 2,4,6-tri(2-piridil)-1,3,5-triazin in 40 mM HCl, 20 mM FeCl3, and 300 mM acetate buffer (pH 3.6) at a ratio of 10:1:1 (v/v/v). A 30 μL aliquot of the ironwood ethanol extract was combined with 270 μL of the FRAP reagent in a 96-well microplate. The mixture was incubated in the dark for 30 min. The absorbance was measured at 593 nm using a UV-Vis spectrophotometer. Trolox (Merck KGaA) was used as the reference standard, and a calibration curve was derived (y = 0.0012x + 0.014; R2 = 0.9981) for concentrations ranging from 100 to 700 μg/mL. The antioxidant activity determined by the FRAP method was expressed as mg Trolox equivalents (TE) per gram of extract.
C. cynocephalus wood termites were obtained from the Termite Rearing Unit, Faculty of Forestry and Environment, IPB University, Indonesia. The termites were adult workers of uniform size with an average weight of ± 0.021 g per termite. The colony was kept in glass containers at room temperature, with humidity regulated by placing the container in a larger vessel partially filled with water. Termites received 250 g of thin wood as a nutritional source until they were used for the experiment.
The no-choice method was used to evaluate the anti-termite and antifeedant activities of the extracts following the procedure described by Upadhyay et al. (2012). Ethanol extracts of the branches at concentrations of 10,000, 15,000, and 25,000 ppm were applied in 0.5 mL volumes to a filter paper (NewStar, 9 cm diameter). The filter paper was then dried at room temperature and weighed. Each concentration was tested in triplicate. The dried filter papers were placed in Petri dishes (9 cm diameter × 2 cm height), and 10 worker termites were introduced into each dish. The Petri dishes were kept in a dark room at room temperature for 7 days. Termites were considered dead if they showed no movement or response to external stimuli. Termite mortality and weight loss of the filter paper were evaluated after 7 days. Mortality was calculated using the following Equation (3):
Where, T1 = the number of live termites before the test; T2 = the number of live termites after the test.
The dose of the test sample (expressed in mg of sample per g of termite body weight) that caused 50% termite mortality was determined to be the LD50 (Suminto et al., 2020). Additionally, the antifeedant activity of the test sample was assessed by observing the decrease in termite feeding on the treated filter paper. After the test, the weight loss of the filter paper was measured and used to quantify the antifeedant activity, where a greater reduction in weight indicated stronger antifeedant properties. Weight loss was calculated using Equation (4):
Where, W1 = weight of filter paper before the test (g); W2 = weight of filter paper after the test (g).
A one-way analysis of variance (ANOVA) was performed to identify significant differences among ulin wood accessions (p < 0.05) for TPC, TFC, antioxidant activity (DPPH and FRAP), and anti-termite activity using IBM SPSS Statistics version 27 (IBM, Armonk, NY, USA). Spearman’s correlation analysis was performed to evaluate the relationships between TPC, TFC, antioxidant activity (DPPH and FRAP), and anti-termite activity (antifeedant and LD50) in ulin wood extract, based on correlation values (r) using R Studio.
3. RESULTS and DISCUSSION
Table 2 summarizes the significant variation in TPC and TFC among the ironwood accessions. The TPC ranged from 202.06 ± 4.31 mg GAE/g DW (accession IX.D.191) to 700.56 ± 12.98 mg GAE/g DW (accession XVI.E.197), while TFC values ranged from 9.90 ± 0.07 mg QE/g DW (IX.D.191) to 16.81 ± 0.37 mg QE/g DW (IX.C.7). These findings highlight significant differences in the bioactive potential of the tested accessions, with the overall phenolic content consistently surpassing flavonoid levels. Statistical analysis using one-way ANOVA confirmed significant differences across accessions (p < 0.05), whereas the Tukey post-hoc test identified groups with no statistically significant differences (p > 0.05).
TPC was determined using the Folin–Ciocalteu method, recognized for its simplicity, reliability, and robustness (Dominguez-López et al., 2023). Among the accessions, XVI.E.197 exhibited the highest TPC (700.56 mg GAE/g), which was approximately three times higher than the lowest TPC observed in IX.D.191 (202.06 mg GAE/g). These results are consistent with prior research by Ibrahim et al. (2023), who reported a TPC value of 416.27 mg GAE/g extract for ironwood sourced from East Kalimantan, comparable to the TPC of accession XX.B.231 (403.33 mg GAE/g) in this study.
The TFC was measured using the aluminum chloride colorimetric method, which reduces interference from non-flavonoid phenolics (Ramos et al., 2017). The highest TFC was observed for accession IX.C.7 (16.81 mg QE/g), whereas IX.D.191 exhibited the lowest value (9.90 mg QE/g). These TFC values are lower than those reported for the stem bark of ironwood from Kutai, East Kalimantan (30.48 mg CE/g) quantified by the aluminum chloride method (Kusuma et al., 2018).
The relatively low flavonoid content in this study may be due to the quantification method, which specifically measures flavonol-type flavonoids such as quercetin, kaempferol, luteolin, apigenin, and myricetin, which form complexes with aluminum chloride (Doloking et al., 2022; Shraim et al., 2021). In contrast, Ahmad et al. (2023) employed a structural elucidation approach using NMR, IR, and Orbitrap Mass Spectrometry to identify other types of flavonoids as well. This approach identified one novel flavonoid and two known flavonols from the ethyl acetate extract of ironwood leaves, namely 7,3’-dihydroxy-3,5,4’-trimethoxyflavone, 7-hydroxy-5,4’-dimethoxyflavone, and 7-hydroxy-3,5,4’-trimethoxyflavone.
These results confirm the substantial bioactive potential of ironwood, with certain accessions such as XVI.E.197 and IX.C.7 emerging as promising candidates for further studies on their potential applications in natural product-based industries.
The antioxidant activity of 15 ironwood accessions was evaluated using two complementary assays: DPPH radical scavenging activity (IC50) and FRAP (Table 3). These assays provide a comprehensive assessment of antioxidant potential, with lower IC50 values indicating stronger radical scavenging activity and higher FRAP values reflecting greater reducing power.
Trolox, used as a reference standard, exhibited the lowest IC50 value (11.96 ± 0.22 μg/mL) and the lowest FRAP value (1.15 ± 0.01 mmol TE/g DW), highlighting its effective radical scavenging ability but comparatively lower reducing power compared to some ironwood accessions. Significant variation in antioxidant activity was observed among the accessions (p < 0.05), with IC50 values ranging from 14.32 to 32.52 μg/mL and FRAP values spanning from 2.40 to 7.68 mmol TE/g DW. This finding aligns with Ridzqya et al. (2024), who observed that genetic differences among ironwood accessions influence the synthesis and accumulation of bioactive compounds, such as phenolics and flavonoids. Statistical analysis using one-way ANOVA confirmed significant differences in antioxidant activity across accessions (p < 0.05), and post-hoc Tukey’s test indicated that certain accessions had comparable activity levels (p > 0.05).
The DPPH assay measured the IC50 values, representing the concentration required to inhibit 50% of the DPPH free radicals. A lower IC50 value indicates a more potent antioxidant activity (Kuspradini et al., 2024). According to a study by Jumina et al. (2019), IC50 values can be categorized into four categories (Table 4). The results revealed that accession XX.A.93 had the strongest DPPH radical scavenging activity (IC50 = 14.32 ± 0.06 μg/mL), while accession IX.D.191 exhibited the weakest activity (IC50 = 32.52 ± 0.21 μg/mL). Overall, all tested accessions belonged to the very strong antioxidant category (IC50 < 50 μg/mL). These results are superior to those reported by Kusuma et al. (2018) for ironwood (IC50 = 44.90 μg/mL), highlighting the potential of the accessions studied here.
| IC50 value (μg/mL) | Category |
|---|---|
| < 50 | Very strong |
| 50–100 | Strong |
| 101–150 | Moderate |
| 250–500 | Weak |
Data from Jumina et al. (2019).
The FRAP assay measures the reducing potential of antioxidants by quantifying their ability to convert Fe3+ to Fe2+. This method is favored owing to its simplicity, cost-effectiveness, speed, and accuracy (Doloking et al., 2022; Prastiwi et al., 2020). Among the accessions, XVI.E.197 demonstrated the highest reducing power (FRAP = 7.68 ± 0.08 mmol TE/g DW), while IX.D.191 showed the lowest activity (FRAP = 2.40 ± 0.03 mmol TE/g DW).
The findings from the FRAP assay highlight the reducing potential of ironwood accessions, which has not been reported previously. Accessions such as XVI.E.197 exhibit promising antioxidant capabilities, suggesting their potential applications in the nutraceutical, pharmaceutical, and functional food industries. Further investigations into the phytochemical composition of these accessions are necessary to identify the bioactive compounds responsible for their antioxidant properties.
The anti-termite activity of the ironwood branch extracts was evaluated against C. cynocephalus using the no-choice method. This method was employed to identify termite activity and evaluate the toxic effects of the extracts. Termite mortality rates and lethal dose (LD50) values were used as key parameters for evaluating the efficacy of the extract (Himmi et al., 2013; Upadhyay et al., 2010). The extracts were tested at concentrations of 10,000, 15,000, and 25,000 ppm, resulting in mortality rates ranging from 10.00% to 96.67% (Table 5). Meanwhile, LD50 values ranged from 0.203 to 0.789 mg/g BW, as determined by the probit analysis of termite mortality (Fig. 1). Statistical analysis using one-way ANOVA revealed significant differences (p < 0.05) in termite mortality rates among the tested concentrations. However, post-hoc analysis using Tukey’s test indicated no significant differences (p > 0.05) between certain groups of accessions with respect to anti-termite activity.
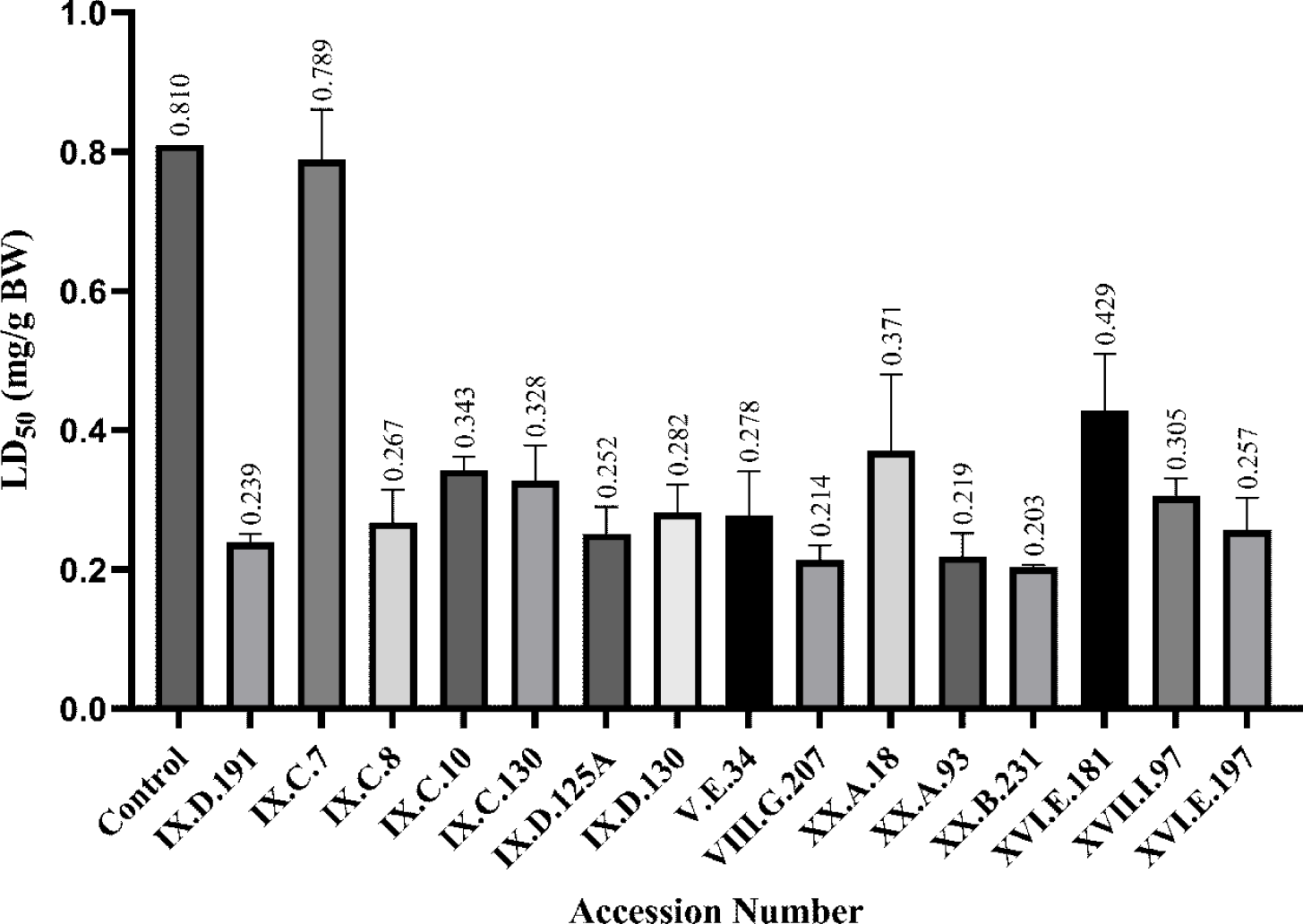
The highest mortality rate was observed for wood accession number XX.B.231 within seven days of testing, with rates of 53.33%, 73.33%, and 96.67% at 10,000 ppm, 15,000 ppm, and 25,000 ppm, respectively. In contrast, the control group had significantly lower mortality rates, ranging from 10.00% to 20.00%. LD50 values further highlighted the potency of the wood extracts. The lowest LD50 value was 0.203 mg/g BW for accession number XX.B.231, indicating a high toxicity. The highest LD50 value of 0.789 mg/g BW was recorded for accession number IX.C.7. Lower LD50 values correlated with greater toxicity to termites, supporting the effectiveness of the extracts (Upadhyay et al., 2010).
These findings indicate that higher extract concentrations and increased toxicity play a significant role in disrupting termite survival. The bioactive compounds in ironwood extracts, such as phenolics and flavonoids, are thought to contribute to their toxicity. These compounds play a defensive role in termites by inhibiting metabolic processes, ultimately leading to mortality (Timotius and Rahayu, 2021). Furthermore, these findings align with previous research by Musman et al. (2020), who reported that higher plant extract concentrations increased termite mortality. Similarly, a study by Amaliyah et al. (2019) demonstrated that ironwood extracts used as preservatives for sengon and rubberwood achieved 100% termite mortality, further supporting the potential of ironwood extracts as an effective pest control agent.
The antifeedant activity of termites was assessed by measuring the weight loss of the paper. Fig. 2 illustrates the decrease in the weight loss of paper treated with ironwood extracts at concentrations of 10,000, 15,000, and 25,000 ppm over a 7-day experiment. The percentage of weight loss observed at 10,000 ppm ranged from 9.17% to 15.40%, while 15,000 ppm concentrations reduced the weight loss to 8.07%–10.25%. The highest concentration, 25,000 ppm, displayed the lowest weight loss range of 4.50%–6.62%. The reduction in weight loss of the sample-impregnated paper was significantly less than that of the control, indicating that the extractive substances in the ironwood extracts acted as an effective antifeedant. Statistical analysis using one-way ANOVA revealed significant differences (p < 0.05) in termite mortality rates among the tested concentrations. However, post-hoc analysis using the Tukey test indicated no significant differences (p > 0.05) in the weight loss of paper across different extract concentrations in terms of antifeedant activity.
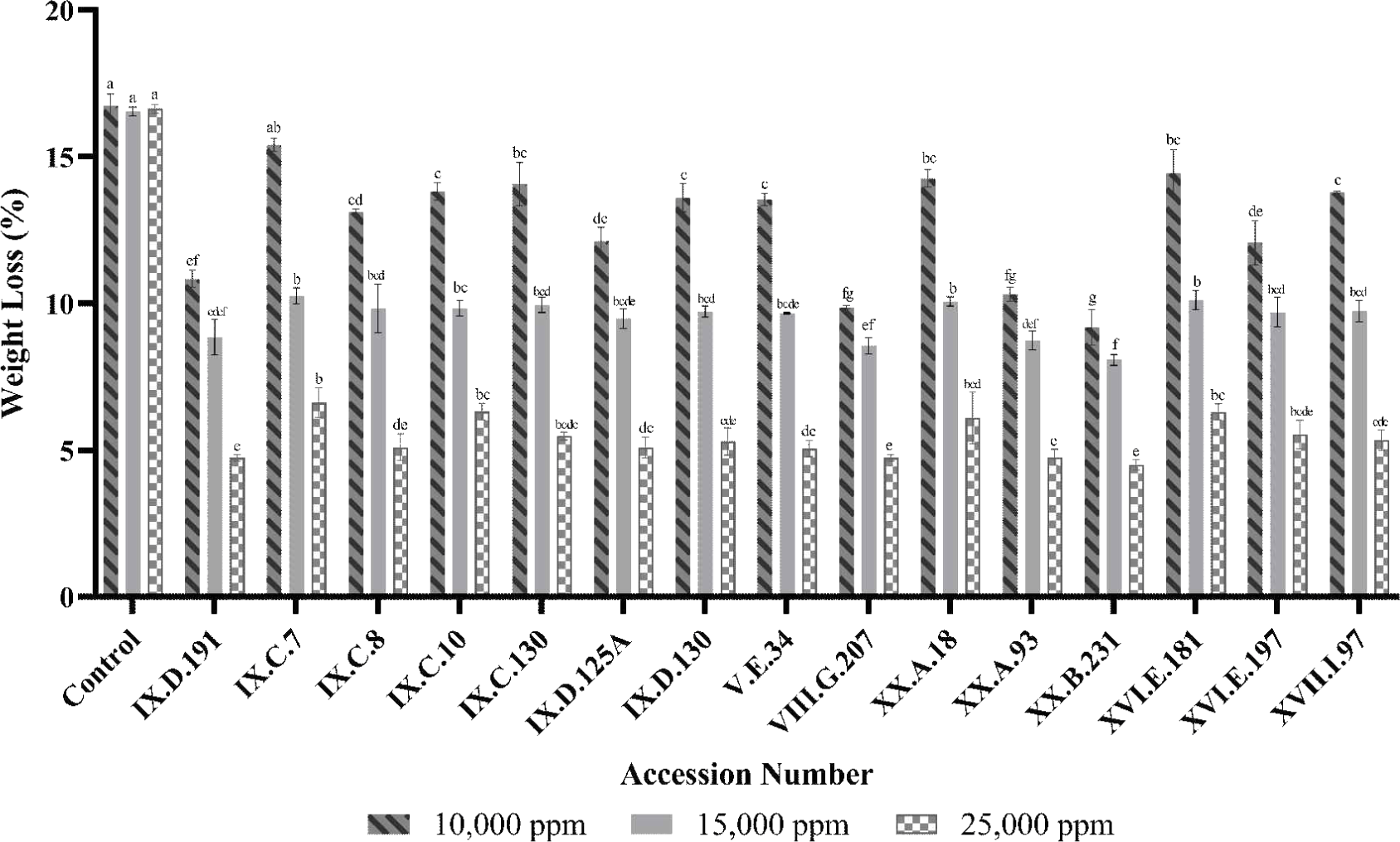
Accession number XX.B.231 exhibited the best antifeedant activity, as evidenced by its lowest weight loss percentage across all concentrations: 9.17% (10,000 ppm), 8.07% (15,000 ppm), and 4.50% (25,000 ppm). These findings suggest a dose-dependent correlation, where higher extract concentrations lead to greater protection by the paper against termite damage. These results align with Amaliyah et al. (2019), who demonstrated that ironwood extracts used as preservatives in sengon wood and rubberwood effectively increased the resistance of the wood against termite attack. In other species, such as Calophyllum inophyllum, Zalsabila et al. (2024) reported that increasing the concentration of its stem bark extract reduced test paper weight loss, indicating improved resistance against subterranean termites.
Spearman’s correlation analysis of ironwood extracts was conducted to evaluate the relationships between TPC, TFC, antioxidant activities (DPPH and FRAP), termite mortality (LD50), and antifeedant activities. The results shown in the correlation matrix (Fig. 3) indicate several significant relationships, emphasizing the multifunctional properties of the extracts.
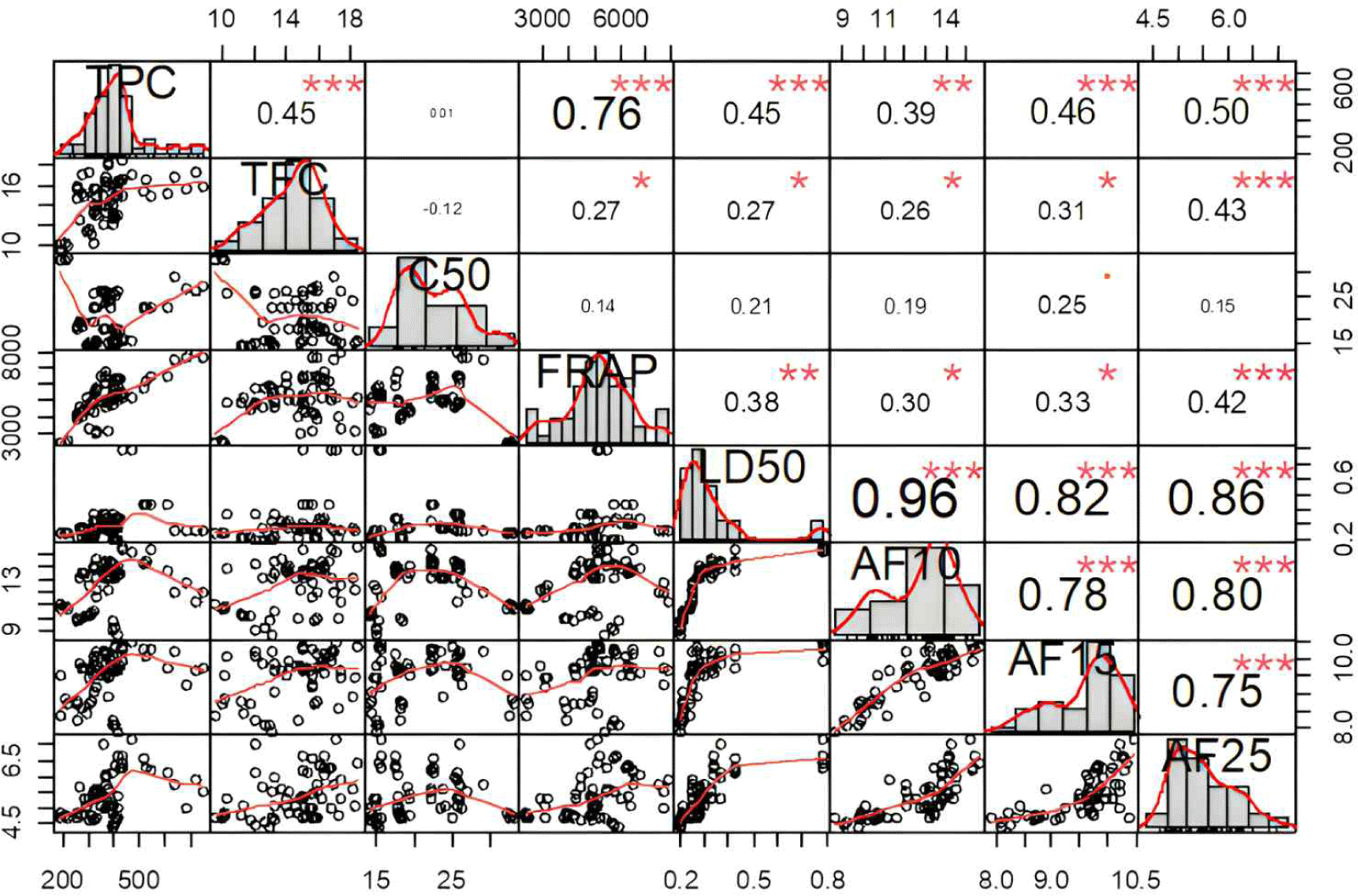
A strong positive correlation was observed between TPC and FRAP antioxidant activity (r = 0.76), indicating that phenolic compounds in the extracts were major contributors to their antioxidant activity, as measured by the FRAP reducing power assay. This result agrees with a prior study that reported a strong correlation between the FRAP assay and the total polyphenol content of torrefied oak wood, indicating that the antioxidant activity of plant extracts is not solely due to phenolics (Nam et al., 2018). Both TPC, measured using Folin-Ciocalteu and FRAP assays, operate through the same mechanism, which allows them to exhibit a strong correlation (Rumpf et al., 2023). However, TPC showed no significant correlation with DPPH radical scavenging activity (r = 0.01), suggesting that phenolics in the extracts may function primarily via electron transfer mechanisms, as measured by FRAP, rather than the hydrogen atom transfer mechanism, which is critical in DPPH assays. Kusuma et al. (2018) found that ironwood stem bark extract exhibited greater superoxide radical scavenging activity than DPPH radicals. These results differ from Hidayat et al. (2018), who reported a strong correlation (r = 0.6) between TPC in the kemenyan resin from Styrax sumatrana and DPPH antioxidant activity.
Similarly, a strong correlation between LD50 and antifeedant activity (r = 0.82 to 0.96) indicated that ironwood extracts with higher toxicity were more effective in inhibiting termites from feeding. This suggests that the extractive substances in ironwood, which are responsible for termite mortality, may also contribute to its antifeedant properties. Moreover, moderate correlations were observed between TPC and both LD50 (r = 0.45) and antifeedant activity, represented by AF10 (10,000 ppm), AF15 (15,000 ppm), and AF25 (25,000 ppm; r = 0.39 to 0.50). This indicates that extracts with higher TPC tend to show greater toxicity and termite deterrence. Previous research has identified secondary metabolites, such as polyphenols, tannins, flavonoids, and anthraquinones, as key factors in the strong anti-termite activity of wood extracts. These results highlight the importance of phenolic compounds in increasing wood resistance to termites, as their presence may disrupt termite digestion and enhance the material’s toxicity (Arsyad et al., 2020; Nkogo et al., 2022). The higher concentration of phenolic compounds has also been connected to the natural durability of wood, with phenolic derivatives like condensed tannins and lignin playing an important role in defense mechanisms (Anouhe et al., 2018; Niamké et al., 2011; Timotius and Rahayu, 2021).
Additionally, a moderate correlation was found between TFC and antifeedant activity at 25,000 ppm (r = 0.43), suggesting its role in termite deterrence. This suggests that extracts with higher flavonoid content may help reduce termite feeding. Although the specific flavonoid compounds in this study have not been identified, previous research has shown that monomeric flavonoids, such as morin, catechin, taxifolin, quercetin, and naringenin, exhibit strong antifeedant activity and toxicity against termites (Mun and Nicholas, 2017). This result aligns with a study by Amaliyah et al. (2019), who reported that ironwood extract, which contains 15.36% phenolics and 11.92% flavonoids, effectively preserves rubberwood and sengon wood, thereby enhancing their durability and reducing their attractiveness to termites.
4. CONCLUSIONS
E. zwageri (ironwood) branch extracts are rich in polyphenol compounds, which exhibit potent antioxidant and anti-termite activities. The results showed notable differences in TPC and TFC, with the highest phenolic and flavonoid contents observed in accession numbers XVI.E.197 and IX.C.7, respectively. Antioxidant assays demonstrated that ironwood extracts exhibited very strong radical scavenging activity against DPPH and high reducing power in FRAP assays. Anti-termite tests revealed significant mortality rates and toxicity effects, with accession number XX.B.231 exhibiting the highest termite mortality and the lowest LD50 values, suggesting potent toxic and defense mechanisms attributed to bioactive phenolic and flavonoid compounds. Furthermore, the evaluation of the antifeedant activity showed a decrease in weight loss of the paper, which increased with higher extract concentrations. This suggests that ironwood extracts are effective in providing a defense mechanism against termite damage. These findings emphasize the potential of ironwood as a rich source of natural extractives and as a viable alternative for termite control and wood preservation. This study also represents a first step toward understanding the genetic basis of wood quality and termite resistance, which could be used to modify other non-endangered woods to produce high-quality wood without exploiting ironwood. Additionally, propagating ironwood and other superior wood varieties could support the sustainability of these highly endangered trees.








