1. INTRODUCTION
Rubber trees (Hevea brasiliensis Muell. Arg.) have been cultivated in Indonesia for over a century. This plant produces latex (rubber sap) for use in tires, adhesives, and other products. Considering the economic value of latex, rubber plantation areas in the country have been maintained and increased annually. Currently, the total rubber tree plantation area in the country has reached 3.67 million ha (Oktavia et al., 2021), making Indonesia the country with the largest rubber plantations. However, beyond its economic age, rubber tree produces less amount of latex. At this point, the rubber tree is commonly cut to prepare for replanting. This process results in a large amount of wood from old rubber trees.
The wood’s creamy color and good working characteristics (de Jesus Eufrade et al., 2015; Teoh et al., 2011) with a density of 0.6 g/cm3, tensile strength perpendicular to grain of 1.4 to 1.9 MPa, hardness of 6000 N, modulus of rupture, and modulus of elasticity of 103 Mpa and 9900 Mpa, respectively, make it desirable for various wood engineered products (Riyaphan et al., 2015). Wood has been extensively used as a raw material in numerous sectors in South East Asia, particularly in Indonesia, Thailand, and Malaysia, including the furniture, kitchenware, and wooden toys industries (Riyaphan et al., 2015). As the final products are primarily exported to other countries, rubberwood-based industries are important for helping countries generate foreign exchange (BPS, 2021). In addition, this sector employs a significant number of laborers (Nandika et al., 2021). However, the wood is highly susceptible to fungal infection owing to the lack of heartwood formation in rubberwood (Ho, 2018; Priyadarshan, 2017; Salman et al., 2020).
George (1985) reported that wood-stained fungi could attack rubberwood immediately one day after it has been felled. Manufacturers of furniture and wooden toys in Southeast Asia are extremely concerned about rubberwood’s susceptibility to fungal stains and mold colonization on its surface (Oldertrøen et al., 2016). Protecting rubberwood from stain fungal attacks is challenging in Indonesia’s tropical climate, with temperatures of 25°C and relative humidity of 80%–100% (Nandika et al., 2021). According to Drewello and Weissmann (1997) and Müller et al. (2001), environmental conditions are favorable for the growth of various wood-inhabiting fungi, including stain fungi. During the rainy season, the water vapor in the atmosphere contains various nutrients, and the fungus specifically colonizes non-durable wood surfaces (Pinto et al., 2019). Economic losses caused by stain fungi attacks on rubberwood used as a raw material in Indonesia’s furniture industry reached over USD 15,246 million in 2018 (Salman et al., 2020). Because many Indonesian furniture manufacturers prefer to use light-colored lumber that is generally less durable, such as rubberwood, for their production processes without using adequate control techniques, it is predicted that this value will increase significantly.
Wood-stained fungi cause discoloration of wood, including rubberwood, significantly reducing its aesthetic value (Nandika et al., 2021). In addition, wood-stained fungi are filamentous fungi that discolor wood surfaces blue, gray, green, or black. Fungal colonization initiates the mineralization process on the wood surface, resulting in discoloration (Nandika et al., 2021; Salman et al., 2020). According to Valiante et al. (2016), melanin production in the conidia during sporulation and pigmentation of the hyphae are the two main causes of discoloration. Aspergillus niger, Aspergillus flavus, and Penicillium citrinum were the three stain fungi that were reportedly isolated from rubberwood in earlier investigations (Oldertrøen et al., 2016). Meanwhile, Salman et al. (2020) reported that at least two stain fungi, Aspergillus aflatoxiformans and A. foetidus, were identified from air-dried rubberwood processed for furniture products in West Java Province, Indonesia. Razali et al. (2016) also identified three groups of fungal isolates, Fusarium equiseti, Mucor irregularis, and Lasiodiplodia theobromae, in rubberwood.
Recently, the authors successfully isolated five isolates of stain fungus from air-dried rubberwood, which is used for furniture products in West Java Province, Indonesia; two of these isolates showed the highest growth rate on wood, namely Aspergillus chevalieri and Paecilomyces formosus. Compared with the other isolates, these two stain fungi caused the worst discoloration, and their effects were evidently different from the natural color of rubberwood. According to the database record of Indonesia Culture Collections (INACC), these isolates may be considered new stain fungus species discovered in the rubberwood industry in the country (Nandika et al., 2021). Hence, a control method for these stain fungi is a potential study.
Catechin is a bioactive substance that is widely present in gambir (Uncaria gambir Roxb.) blocks and is recognized as a complex flavonoid from the polyphenol group (Anggraini, 2011; Apea-Bah et al., 2009; Taniguchi et al., 2007). The antimicrobial activity of catechin has been known for decades (Kim et al., 2002, 2020), including its activity against bacterial colonies in male Wistar rats (Dewi et al., 2018), Staphylococcus aureus (Merta et al., 2013), Streptococcus mutans (Pambayun et al., 2007), and Enterococcus faecalis (Katu et al., 2016). Recently, the chemical components of catechin extracted from gambir and its bioactivity against the wood-decaying fungus Schizophyllum commune have been studied. Catechin has five chemical components: 1,2-benzenediol; catechol; 1,3,5-benzenetriol; dimethyl terephthalate; and terephthalic acid. These compounds demonstrated the ability to inhibit the growth of S. commune (Nandika et al., 2019). However, the bioactivity of catechins against wood-stained fungi has not yet been reported.
Therefore, investigating the bioactivity of catechins extracted from gambir against fungi that cause severe rubberwood discoloration would be interesting. This is because practically all wood preservatives used today to prevent wood-staining by fungi are synthetic substances that are potentially detrimental to the environment and human health. Furthermore, every synthetic wood preservative sold in Indonesia and other Southeast Asian countries is an expensive imported product (Nandika et al., 2019). Hence, this research will hopefully contribute considerably to the development of environmentally friendly organic fungicides, which are needed to prevent wood-staining fungal attacks on various wood species used in the Indonesian wood industry. Furthermore, gambir is recognized as an indigenous Indonesian product. Therefore, utilizing this commodity as an active ingredient in natural wood preservatives could increase their economic value.
2. MATERIALS and METHODS
Air-dried rubberwood boards, used as furniture products, were provided by one of the largest furniture industries in Bekasi, West Java Province, Indonesia. The furniture industry prepared wood from natural rubber trees in Palembang, South Sumatra Province, Indonesia, and stopped producing latex after 25 years. The dimensions of the boards prior to wood sample preparation and treatment were 35 mm thick by 70 mm wide by 400 mm in length, with their length in a direction parallel to the grain. They consisted mainly of sapwood; the sampling method was based on the methodology of ISO 3129 (ISO, 2012). The mean moisture content and density of the air-dried boards were 15.66% and 0.642 g/cm3, respectively (ISO, 2014). The rubberwood was transferred to the laboratory for further procedures and stored in dark plastic bags.
Gambir (Uncaria gambir Roxb.) cylindrical blocks with a diameter of 2.07 cm, thickness of 0.53 cm, and height of 2.23 cm, were obtained from Talang Maua Village, Mungka District, Lima Puluh Kota Regency, West Sumatera Province.
The catechin extraction method was as described by Nandika et al. (2019). The gambir blocks were milled and screened using 100 mesh screeners. Catechin was extracted according to TAPPI T207 cm-99 (TAPPI, 1999) using 1:5 (w/v) hot water for 3 h. Hot water extraction was performed to separate the water-soluble chemicals that led to contaminants in the extract. In contrast to methanol at ambient temperature or boiling point, Sousa et al. (2008) found that hot water extraction was more effective in extracting phenolic antioxidant compounds. The extracts and tannins were separated by repeated sedimentation in cold water (20°C) after the extraction product was precipitated for 24 h. The dried filtrate was macerated for 4 h using ethyl acetate 50% and filtered through a Whatman 42 filter paper (GE Healthcare, Buckinghamshire, UK). The obtained filtrate was dried with a spray dryer at an inlet temperature of 175 ± 5°C and an outlet temperature of 60 ± 5°C.
The moisture content of the extracted catechin was analyzed, and the particle size of the catechin powder was determined using a particle size analyzer (PSA LA-960, Horiba Instruments, Irvine, CA, USA). The texture, color, and odor of the catechins were determined by organoleptic assessment according to the Indonesian Standard (SNI 01-2346-2006; BSN, 2006).
The A. chevalieri IPBCC 22 153 isolates were supplied by IPB University Culture Collection (IPBCC, Bogor, West Jaya Province, Indonesia) and were cultivated on malt agar (supplied by HiMedia Laboratories, Mumbai, India) under temperature of 4°C. The isolates were originally isolated from air-dried rubberwood prepared for furniture component production in Bekasi, West Java Province, Indonesia, in 2019 (Nandika et al., 2021) and then handed over to IPBCC. The medium used to cultivate A. chevalieri fungus was introduced into the autoclave for sterilization at a temperature of 121°C for 15 minutes. In addition, 20 mL of medium was poured into each Erlenmeyer 100 mL and incubated for one week on an Eyela Multi Shaker MMS-210 at 120 rpm at room temperature (28 ± 2°C) with a relative humidity of 75 ± 5%.
Wood samples with dimensions of 8 mm thickness × 20 mm width × 30 mm length, with their lengths parallel to the grain and fulfilling ASTM D 4445-03 (ASTM, 2008), were sterilized by the steam method using an autoclave (GEA Medical, YX-24LDJ, Jiangyin Binjiang Medical Equipment, Jiangsu, China) for 15 min. The wood samples were first introduced into a stainless-steel autoclave as a closed system, and a vacuum (50 bar) was created and maintained for 15 min. Each of the prepared catechin solutions in dimethyl sulfoxide (DMSO, Sigma Aldrich, St. Louis, MO, USA) solvent (0% control/untreated, 6%, 9%, 12%, and 15% w/v, respectively, with six replications) was then introduced by the pressure difference (into an autoclave), and the pressure was increased to 2.5 psi for 2 h. Finally, the pressure was removed, and the wood samples were extracted from the remaining solution. Each catechin treatment, as well as the untreated and positive controls, had six replicates. After impregnation, wood samples were left at 28 ± 2°C with a relative humidity of 75 ± 5% for 24 h.
The WPG of each catechin-treated wood sample was determined based on the variations in the oven-dried weight before and after impregnation of the catechin solution, as calculated using Equation (1):
where W0 is the oven-dried weight of the specimens before the catechin treatment, and W1 is the oven-dried weight of the specimens after the catechin treatment.
The medium used to cultivate A. chevalieri fungus was prepared using malt extract agar medium and was introduced into an autoclave for sterilization of all wood samples. Twenty milliliters of the medium were placed in each petri dish (d = 9 cm) used for the test. Six wood samples were used for each catechin concentration, and one treated wood sample was placed in each petri dish with one negative control (untreated) sample.
The A. chevalieri fungus was inoculated by applying a 0.25 mL spore suspension 106 cm3 to the surface of the agar medium in a petri dish and to the wood samples. Following inoculation, each petri dish was stored in an incubator for 3 weeks at a temperature of 28°C and a relative humidity of 75%. The catechin treatment efficacy was evaluated after 1, 2, and 3 weeks by visually analyzing fungal growth on the top surface of the wood samples in line with the scale shown in Table 1 (ASTM D 5590:2017; ASTM, 2017).
The CIELab method was employed to determine the discoloration of wood samples due to infection by the stain fungus A. chevalieri. This method involves directly measuring the L*, a*, and b* values in photographs. Images were obtained using a stereomicroscope (Olympus SZ2-ILST, Olympus, Tokyo, Japan) and analyzed using the Corel Draw X8 application (Afshari-Jouybari and Farahnaky, 2011; Gahruie et al., 2017). Staining values were obtained and indicated by the signs L*, a*, and b*. L* represents lightness, with a value range from 0 to 100 (black to white); a* represents colors, with +a* ranging from 0 to 80 corresponding to red and –a* ranging from –80 to 0 corresponding to green; and b* represented colors, with +b* ranging from 0 to 70 corresponding to yellow and –b* ranging from –70 to 0 corresponding to blue (Christie, 2015). Each sample was assessed at five points, and the average values were used for analysis. The color change (ΔE) was calculated using the CIELab method (HunterLab, 2012) and Equation (2):
where ΔE: color change; ΔL: difference in L* values between compared samples; Δa: difference in a* values between compared samples; and Δb: difference in b* values between compared samples. The color changes can be classified as shown in Table 2.
Adapted from Hrčková et al. (2018) with permission of NC State University.
Adapted from HunterLab (2012) with permission of Hunter Associates Laboratory.
Microscopy of the stain fungi mycelia on the wood sample surface after 1, 2, and 3 weeks inoculation was conducted using a scanning electron microscope (SEM)-EDS energy-dispersive X-ray spectrometer (JSM-IT200, JEOL, Tokyo, Japan). Prior to this, specimens 5 mm in length × 5 mm in width × 2 mm in thickness from each of the catechin concentration treatments (0% or control, 6%, 9%, 12%, and 15%) were covered by galvanized gold deposition using an MC1000 ion sputter (FEI, Tokyo, Japan) operating at a current of 5 mA for 45 s.
SPSS software version 24 was used for statistical analysis and processing of the results. SPSS software version 24 was used for statistical analysis and processing of the results. The responses of WPG and color change after particular treatments were analyzed using a one-way analysis of variance. The bioactivity data and discoloration after exposure to the stain fungi were analyzed using a two-way analysis. If any significant (p ≤ 0.05) features were in the data, the analysis was continued using Duncan’s multiple range test.
3. RESULTS and DISCUSSION
Through a multilevel extraction method using hot water (70°C) and ethyl acetate (1:10 w/v), we extracted catechin from gambir with a yield of 35.7%. This yield was similar to the extraction yield reported by Nandika et al. (2019) using the same method, which was 33.5%. In addition, Sucipto et al. (2020) reported relatively similar results with gambir blocks containing 31.1% catechin. Rismana et al. (2017) attempted to extract catechin from gambir blocks using 50% and 96% ethanol, with 66.8% and 76.4% yields, respectively. The size of the gambir block, temperature and duration of the extraction, and type of solvent utilized may have affected the yield. However, according to several studies (Failisnur et al., 2018; Rahman et al., 2018; Yeni et al., 2014), the use of ethyl acetate for gambir extraction is considered the optimal choice based on the variables of time and cost-effectiveness. Supporting the results reported by Nandika et al. (2019), the physical characteristics of extracted catechin in this present study were in the form of a fine yellowish-white powder and were odorless, with a water content of 9.1%. Based on PSA analysis, the size of the catechin powder was 6.6 ± 0.1 μm.
Fig. 1 shows the WPG of catechin in the wood samples for each concentration of catechin solution. Analysis of variance revealed that the concentration of the catechin solution affected the WPG of catechin. Duncan’s test revealed that the highest WPG was obtained in the wood sample treated with the 12% concentration solution, followed by the samples impregnated with 15%, 9%, and 6% catechin. In addition, the WPG catechin levels in wood samples treated with 9% and 15% catechin solutions were not significantly different (p < 0.05).
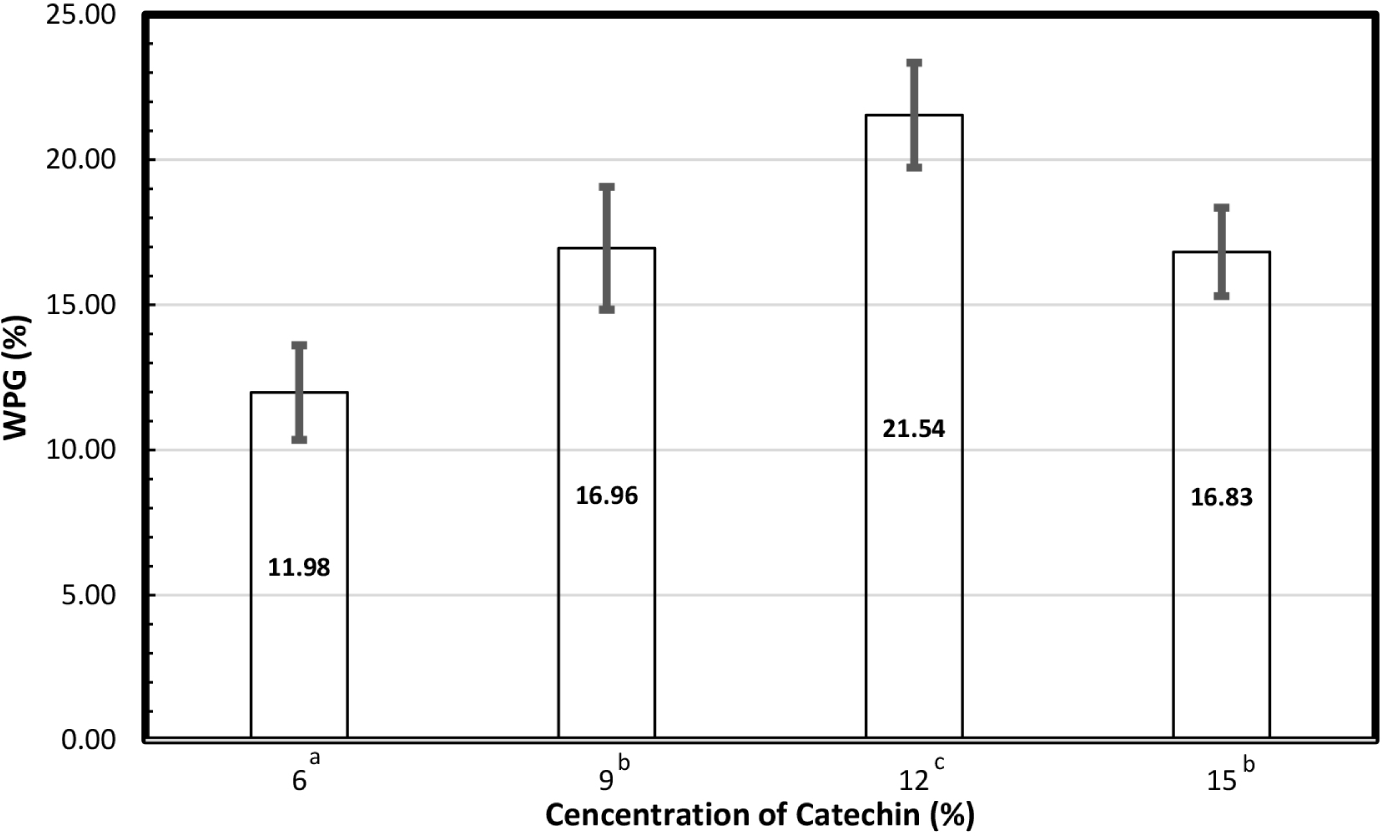
The WPG of catechins in this study was almost that reported by Nandika et al. (2019), who impregnated the same wood species at concentrations of 6%, 9%, and 12%. According to Gabrielli and Kamke (2010), the WPG provides data on the extent to which an impregnating substance can penetrate the cellular structure of wood; higher WPGs indicate greater penetration. A significant amount of catechin was deposited in the wood samples, as evidenced by the high WPG values. Specifically, catechin was easily impregnated into rubberwood using a vacuum followed by a pressure procedure in a closed system. The high molecular weight of the compound was attributed to a decline in the WPG in wood samples treated with more than 12% catechin solution (Nandika et al., 2019). The treatment of wood was affected by several variables, including the solubility of the impregnant in water, the molecular weight or size, and the viscosity of the impregnant solution (Pittman et al., 1994). High concentrations increased viscosity, decreasing the wood permeability (Stamm, 2002). This resulted in the impregnant being unable to penetrate the wood through the pores and pits. Furthermore, WPG at 15% was lower owing to its higher viscosity and decreased permeability.
The efficacy of catechin in inhibiting the stain fungi A. chevalieri was shown by the low average score of the stain fungi growth on the surface of the wood samples treated with the catechin solution at concentrations of 6%, 9%, 12%, and 15%, after 1, 2, and 3 weeks of incubation. Catechin treatment of wood samples at the abovementioned concentrations induced a mean score of stain fungi growth in the range of 0 (no growth of fungi on the wood sample) to 2.17 (less than 30% of the wood sample area covered by fungi). The score of stain fungal growth on wood samples treated with 12% and 15% catechin solutions was 0. Specifically, catechin treatment at 12% and 15% concentrations demonstrated high antifungal activity, protecting unseasoned rubberwood from fungal attack over time. Based on a study by Toyoshima et al. (1994), the mechanism of the effects of catechin on fungi using electron microscopy suggested that catechin attacked the cell membrane and caused lysis of conidia and hyphae. In contrast, the score for stain fungi growth on the untreated samples was 4 (Fig. 2).
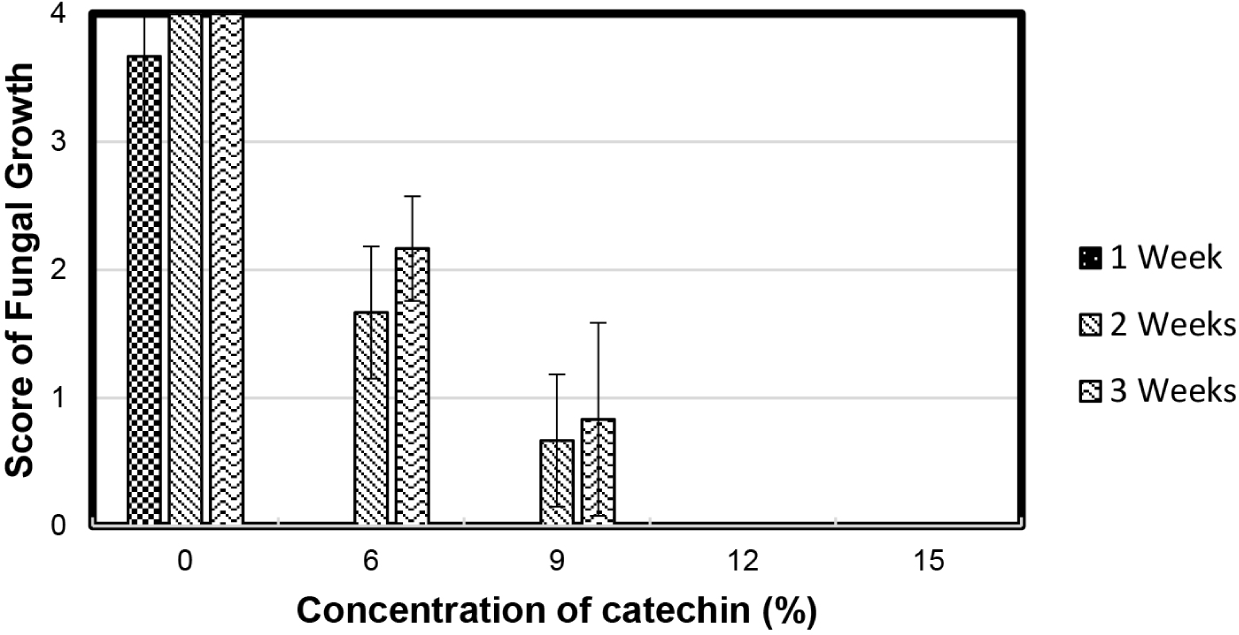
The analytical statistics show that the untreated wood samples (0% catechin concentration) had the highest degree of fungal growth, followed by wood samples treated with 6%, 9%, 12%, and 15% catechin solution, respectively (p ≤ 0.05). As the catechin concentration increased, the degree of fungal growth (coverage) on the treated wood samples decreased. The stain fungus grown on untreated wood samples appeared earlier than on 6%-treated catechin samples, followed by 9%-treated catechin samples (p ≤ 0.05). Specifically, catechin treatment delayed the germination time of fungi in the treated samples. Therefore, the untreated wood samples were more susceptible to fungal attack than the catechin-treated wood samples. Notably, the average fungal growth score of the 15% catechin-treated wood samples (0) was lower than that of the 9% catechin-treated wood samples (0.8), despite the insignificant difference in the WPG of both treated samples. Consequently, we argue that the active compound in the 15% catechin-treated wood samples was mainly trapped on the wood sample surface because of its high viscosity. The active compound in the 9% catechin-treated wood sample penetrated more and was distributed into the inner part of the wood sample, owing to its lower viscosity. This finding is consistent with that of Pittman et al. (1994), who identified several factors affecting the effectiveness of wood treatment, including the viscosity, molecular size, and molecular weight of the impregnants.
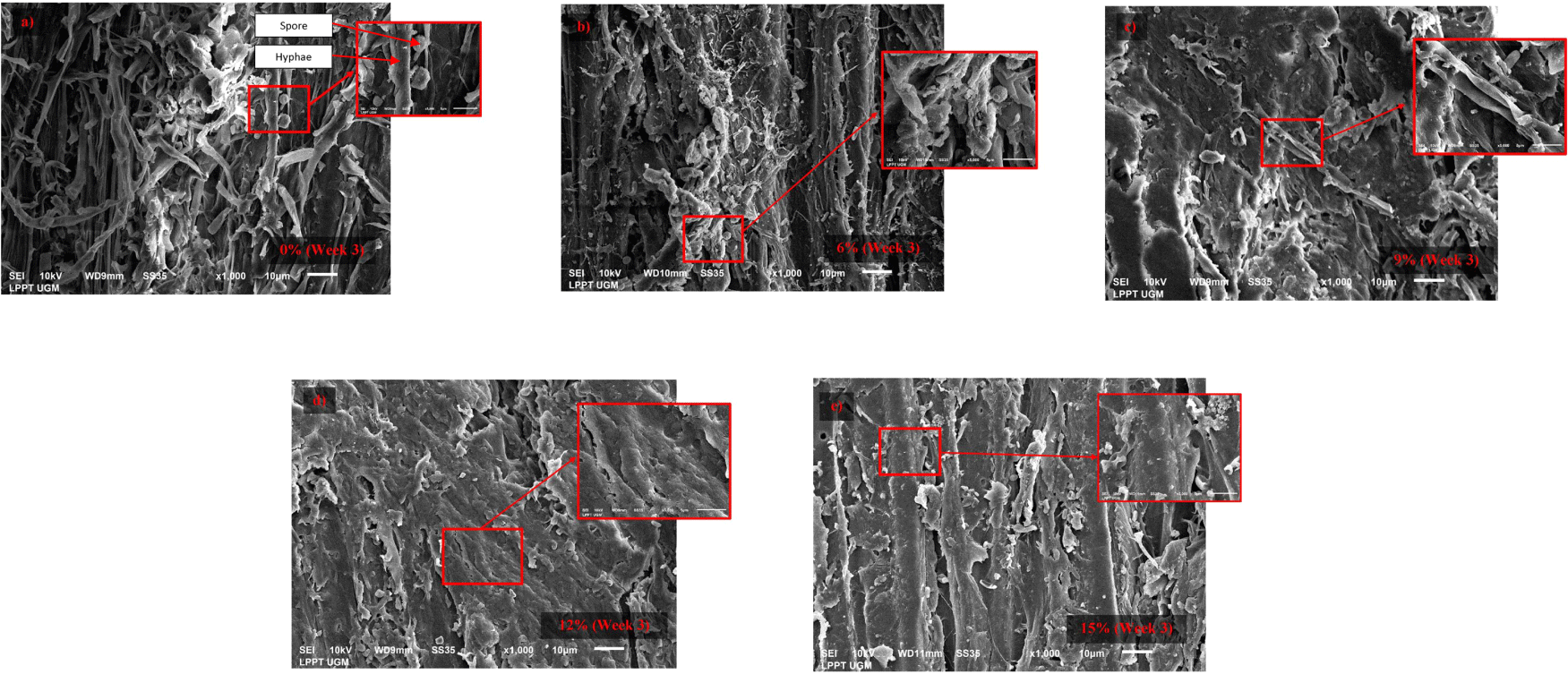
Fig. 3 shows the micrograph of the stain fungi mycelia on the untreated wood samples (0% catechin concentration) and the wood samples treated with catechin solutions (6%, 9%, and 15%) after 3 weeks of incubation. Based on SEM analysis, the untreated wood samples were found fully covered with stain fungi mycelia from the first visual assessment in the first week of exposure, whereas the 6% and 9% catechin-treated wood samples were covered to a very limited extent in the second and third weeks of exposure. Even, no fungal growth was observed in the 12% and 15% catechin-treated wood samples. As the catechin concentration increased, the degree of stain fungi growth on the wood sample surface decreased, compared to the untreated wood samples, until 3 weeks of incubation. Therefore, it can be concluded that the catechin solution was effective against the fungus Aspergillus chevaileri.
The results showed that the natural color of rubberwood (without fungal attack) was yellowish white, with an average value of L* = 73.6, a* = 4.5, and b* = 21.2. Regarding the natural color of rubberwood, Jiang et al. (2020) reported almost the same L*, a*, and b* values of 80.94, 4.37, and 21.69, respectively. Meanwhile, after inoculation with the stain fungi for 3 weeks, the color changed to darker with an average value of L = 41.5, a = 2.3, b = 10.5 for the 0% catechin concentration as a control.
Based on the results shown in Table 3 and Table 4, the color of the treated wood samples, compared to the 0% catechin sample, was significantly different in terms of their L*, a*, and b* values. The wood samples with a higher concentration of catechin solution had higher lightness (L* value) than those with a lower concentration. In addition, wood samples impregnated with catechins tended to have a redder color (higher a* value) than those impregnated with low concentrations, which tended to be greenish (lower a value). However, the variation in the concentration of catechins did not affect the b* value, which was more yellowish than that of the untreated wood samples. In summary, there was a significant difference in color (ΔE) between the untreated wood samples (control) and wood samples impregnated with catechin solutions. This finding is relevant to the study by Hadi et al. (2020, 2022), who reported that any chemical treatment of wood could potentially change its natural color. However, no color differences were observed among the wood samples treated with catechin solutions at concentrations of 9%, 12%, and 15%.
| Catechin concentration(%) | L* | a* | b* | △E |
|---|---|---|---|---|
| 0 | 41.5 | 2.3 | 10.5 | 0.0 |
| 6 | 69.5 | 4.5 | 19.5 | 26.6 |
| 9 | 72.8 | 5.8 | 23.0 | 33.8 |
| 12 | 74.7 | 6.4 | 21.3 | 35.2 |
| 15 | 75.9 | 6.6 | 22.6 | 36.8 |
| Response | Catechin impregnation |
|---|---|
| L* value | *** |
| a* value | *** |
| b* value | *** |
| △E discoloration | *** |
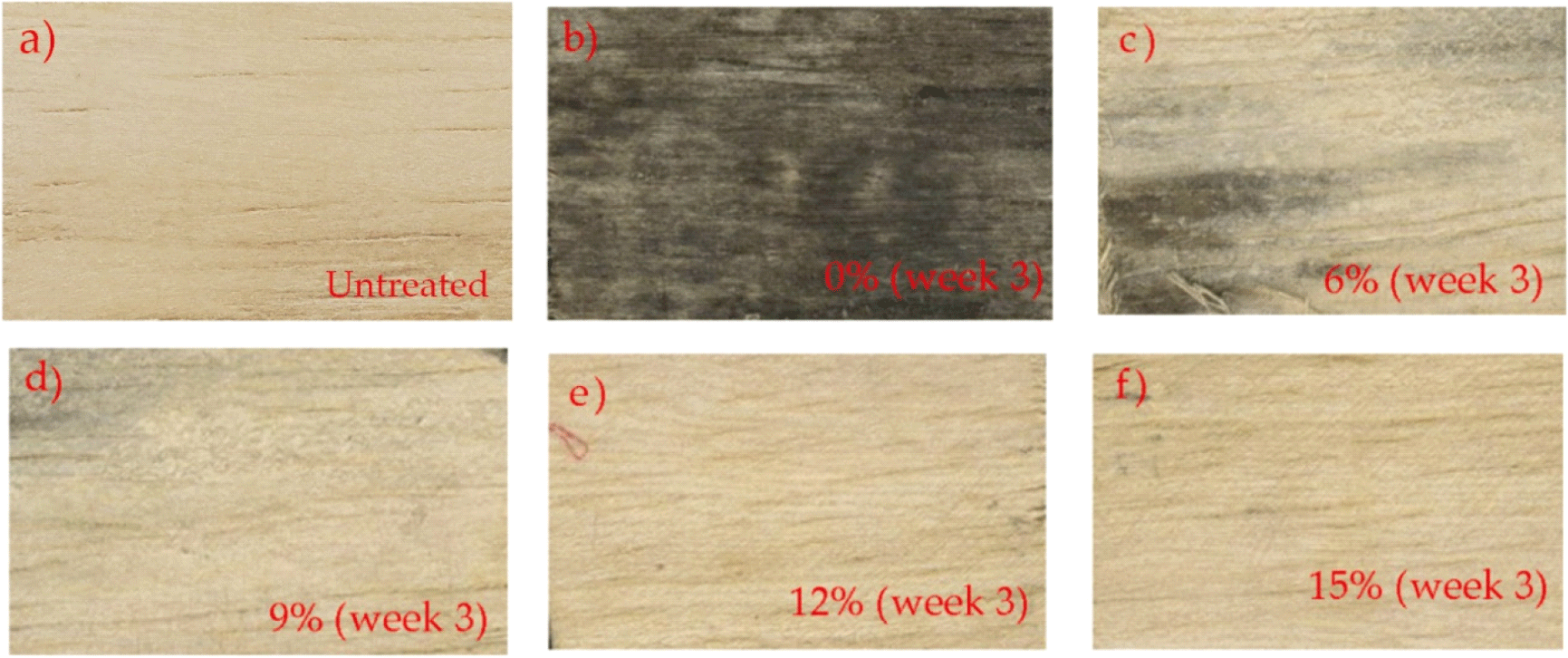
Nandika et al. (2021) reported that stain fungi A. chevalieri caused severe discoloration on air-dried rubber wood with L* = 54.35, a* = 10.8, and b* = 18.15 and ΔE = 27.60 after 4 weeks of incubation. In this study, the treated wood samples exhibited ΔE of more than 12 (Table 3). Based on the color-change classes shown in Table 2, it can be concluded that the color of the treated wood samples was significantly different from that of the untreated wood samples. In particular, the treated wood samples showed less discoloration than the untreated wood samples and even demonstrated no discoloration, as exhibited in the wood samples treated with catechin solution at concentrations of 12% and 15% (Fig. 4). Based on these observations, at concentrations of 12% and 15%, catechins effectively prevented the growth of the stain fungi. Furthermore, there was no significant discoloration of the wood surfaces treated with either concentration. Toyoshima et al. (1994) reported that catechin attacks the cell membrane and causes lysis of conidia and hyphae. As shown in Table 3 and Fig. 4, the catechin solution treatment inhibited the growth of A. chevalieri on air-dried rubberwood. Consistent with these findings, the treatment also demonstrated a significant performance in preventing wood discoloration. Therefore, the catechin solution can be proposed as an alternative protective agent for rubberwood-based products against wood-stain fungi.
4. CONCLUSIONS
The physical characteristics of extracted catechin in this present study were a fine yellowish-white powder (6.6 ± 0.1 μm) and odorless, with a water content of 9.1%. Using the vacuum-pressure method in a closed system, the catechin solution in DMSO at concentrations of 6%, 9%, 12%, and 15% could penetrate the wood samples, as reflected by the high WPG ranging from 12% to 21%.
By vacuum pressure impregnation, the catechin extracted from gambir in DMSO solution showed a protective effect on air-dried rubberwood against the stain fungus A. chevalieri. Up to a catechin concentration of 15%, the higher the catechin concentration, the lower the fungal growth. After 3 weeks of incubation, the wood samples treated with 12% and 15% catechin concentrations showed no growth of the stain fungi (score = 0) and no discoloration. Meanwhile, the untreated wood samples showed severe infestation with the stain fungi (score = 4), simultaneously demonstrating significant discoloration. A catechin solution is proposed as an alternative protective agent for rubberwood-based products against wood-staining fungi.








