1. INTRODUCTION
Candida spp. are resident fungi isolated from skin, digestive tract, vagina, and oral cavity (Hedderwick and Kauffman, 1997). They are generally harmless to humans, but their overgrowth can cause infectious diseases. Among Candida spp. causing pathogenic infections, Candidaalbicans is the species most frequently isolated from clinical specimens (Kumamoto and Vinces, 2005). Candida spp., including C. albicans, exist in up to 75% of microbiota in the oral cavity (Calderone and Clancy, 2011), and oral candidiasis can occur due to overgrowth or infection (Epstein, 1990). Vulvovaginal candidiasis is a common disease suffered by approximately 75% of women at least once in their lifetime (Sobel, 1992, 1997). It has been reported that more than 90% of cases of vulvovaginal candidiasis are caused by C. albicans (Sobel, 2007). Candida spp. have also been reported to cause bloodstream infections in patients in intensive care units (Stover et al., 2001; Wisplinghoff et al., 2004), and such infections lead to prolonged hospitalization and high mortality (Falagas et al., 2006). According to surveys, candidiasis is the second leading cause of death due to nosocomial infections (Beck-Sagué et al., 1993).
Fluconazole, intraconazole, and voriconazole, which are azoles, and amphotricin B, a polyene, are used for treating C. albicans infection (Garcia-Cuesta et al., 2014; Pfaller et al., 2009). However, recent studies have reported the identification of fluconazole-resistant C. albicans strains (Raschig et al., 2023; Xiang et al., 2013; Zhao et al., 2013). As the need for safe antifungal agents increases, plant-derived materials are being actively investigated as a source of such agents. Between 1966 and 1994, 115 papers were published in PubMed on the antimicrobial activity of medicinal plants, but during the decade between 1995 and 2004, this number increased sharply to 307 (Ríos and Recio, 2005). Many plants used in traditional medicine contain natural bioactive agents that exert health-promoting activities with little or no side effects. These traditional medicinal plants are known to have various secondary metabolites such as tannins, terpenoids, alkaloids, and flavonoids with antimicrobial activities (Cowan, 1999). Many extracts and chemicals from plants showed antifungal activity (Adfa et al., 2020; Hidayat et al., 2019), antibacterial activity (Ham et al., 2020; Mun et al., 2021), and antibiofilm activity (Ham and Kim, 2018, 2019, 2022).
Several plant components have been shown to act synergistically with antifungal agents and to exert therapeutic effects against fungal infections with the benefits of broad efficacy, safety, reduced toxicity, and reduced antifungal resistance (Augostine and Avery, 2022; Lee et al., 2021; Ogidi et al., 2021). The use of two antifungal agents that act synergistically is particularly useful and effective because they can increase both the rate and the degree of sterilization (Mukherjee et al., 2005), while each agent may also have a different mechanism of antifungal activity (Johnson et al., 2004; Wagner and Ulrich-Merzenich, 2009). Recently, with the publication of an increasing number of reports on the azole resistance of C. albicans, it has become more important to increase antifungal activity by establishing appropriate mixtures of new antifungal substances and existing antifungal agents (Rosato et al., 2008). In a previous study (Nweze and Eze, 2009), Ocimum gratissimum leaf ethanol extract showed synergistic antifungal activity with ketoconazole against clinically isolated C. albicans. CaThi, a plant-derived peptide component, is known to have a synergistic effect by binding with fluconazole and affecting the invasion of C. albicans plasma membrane (Taveira et al., 2016). Basil essential oil and its main single compound, geraniol, have also been reported to exert synergistic effects on C. albicans and Cryptococcus neoformans upon mixing with antifungal agents (Cardoso et al., 2016). In addition, our previous studies showed that combinations of Magnoliae Cortex extract with Syzyii Flos extract (Yoon and Kim, 2021) or Phellodendri Cortex extract (Na and Kim, 2022) showed synergistic antifungal activity.
Many studies have been conducted on the synergistic activities that can be obtained by mixing plant-derived compounds or plant essential oils with antifungal agents. However, studies demonstrating synergistic activity upon mixing plant extracts are limited. In this study, antimicrobial activities against Staphylococcusaureus, and Malasseziapachydermatis were also considered, and synergistic antifungal combinations of selected herbal extracts were screened comprehensively and systematically.
2. MATERIALS and METHODS
C.albicans KCTC 7965, M. pachydermatis KCTC 17008, and S. aureus KCTC 1916 were purchased from Korean Collection for Type Cultures at the Korea Research Institute of Bioscience and Biotechnology (Jeongeup, Korea). Both fungi were mixed with 15% glycerol and S. aureus was mixed with 25% glycerol and stored at –80°C.
A library with extracts from 280 edible plants from a previous study (Yoon and Kim, 2021) was used here. The extract preparation method constituting the library was described in a previous study (Ham and Kim, 2018). Yeast extract peptone dextrose medium (YPD), Sabouraud dextrose medium, and RPMI-1640 medium were made in accordance with a previous study (Yoon and Kim, 2021). Modified Dixon (mDixon) medium was prepared with 3.6% malt extract, 2% desiccated ox bile, 1% Tween 40, 0.6% peptone, 0.2% glycerol, and 0.2% oleic acid. Tryptic Soy Broth (TSB) medium was prepared with 3% TSB (REF: 211825; Becton Dickinson, Franklin Lakes, NJ, USA). mDixon agar plates, Sabouraud dextrose agar plates, TSB agar plates, and YPD agar plates were prepared by adding 1.5% agar to the corresponding medium.
A disk with the plant extract for the paper disc diffusion method was made in accordance with a previous study (Yoon and Kim, 2021). M. pachydermatis was pre-cultured in mDixon medium at 30°C for 4 days. The pre-cultured cells were diluted to Abs600 = 1.5 using mDixon medium, and spread evenly on mDixon agar plates with 0.1 mL of diluted cells. A paper disk with 5 mg of plant extract was placed on the plate smeared with cells (Yoon and Kim, 2021). After culturing for 48 h at 30°C with 5 mg of extract, it was judged that there was an antifungal effect when a growth inhibitory ring of 1 mm or more had formed on the paper. The width and length of the growth inhibitory ring were measured in millimeters. Six values measured in two independent experiments were averaged.
S. aureus was pre-cultured in TSB medium at 37°C for 24 h. The pre-cultured strain was diluted to Abs600 = 1.0 using TSB medium, and 0.1 mL of diluted cells were evenly spread on TSB agar plates. A paper disk with 5 mg of plant extract was placed on the plate smeared with cells. After culturing for 24 h at 37°C, it was judged that there was an antibiotic activity when a growth inhibitory ring of 1 mm or more had formed on the paper. The width and length of the growth inhibitory ring were measured in millimeters. The four values measured in two independent experiments were averaged.
The activity of inhibiting the growth of C. albicans according to the concentration of plant extracts and the synergistic inhibition of C. albicans growth by the combination of two plant extracts were evaluated in accordance with the procedure in a previous study (Yoon and Kim, 2021). Powdered extracts and chemicals were dissolved at 100 times the concentration to be tested. The combinational antifungal activity of each 50 μL extract or chemical was evaluated in 5 mL of YPD medium.
3. RESULTS and DISCUSSION
In a previous study (Yoon and Kim, 2021), 17 plant extracts were selected by evaluating the antifungal effect on C. albicans using the paper disk diffusion method. In this study, it was evaluated whether these 17 selected plant extracts exhibited antifungal and antibacterial activity against M. pachydermatis, another animal skin fungus, and S. aureus, a skin bacterium, respectively (Table 1). Additional antifungal activity against M. pachydermatis and antibiotic activity against S. aureus provide broad applications against skin fungi and bacteria.
Among the 17 tested plant extracts, 16 of them (with the exception of Polygalae Radix) exhibited antifungal activity against M. pachydermatis. Similar to the results for C. albicans (Yoon and Kim, 2021), the inhibited ring size of Cinnamomi Cortex was 42.3 mm, showing the greatest inhibitory activity. With the exceptions of Cinnamomi Ramulus, Cocculi Radix, and Polygalae Radix, larger inhibitory ring sizes were observed in M. pachydermatis than in C. albicans.
In the evaluation of the antibiotic activity of 17 plant extracts against S. aureus, Cocculi Radix, Polygalae Radix, and Rhizome of Kaempferia galangal extracts showed no antibiotic activity. Among the 14 extracts with antibiotic activity against S. aureus, Cinnamomi Cortex showed the greatest activity with an inhibitory ring of 22.3 mm, and Syzygii Flos showed the second largest inhibitory activity with 21.8 mm, similar to the results of C. albicans and M. pachydermatis.
According to the results of Table 1 and the antifungal activity on C. albicans in a previous study (Yoon and Kim, 2021), Cinnamomi Cortex showed the greatest growth inhibitory activity against all tested three strains, while Syzygii Flos and Magnoliae Cortex also showed excellent antimicrobial activity.
Cocculi Radix, Polygalae Radix, and Rhizome of Kaempferia galangal, which had no antibiotic activity against S. aureus, were excluded from additional experiments because, in terms of skin hygiene, it would be highly beneficial to inhibit the growth of all three tested strains simultaneously.
Among 14 selected plant extracts, the antifungal activity of Cinnamomi Cortex, Cinnamomi Ramulus, Magnoliae Cortex, and Syzygii Flos was evaluated against C. albicans in a previous study (Yoon and Kim, 2021). The antifungal activity of the remaining 10 plant extracts against C. albicans was measured in this study. Among them, the extracts of Amomi Tsao-ko Fructus, Cnidii Rhizoma, Flower of Rosa multiflora, and Sanguisorbae Radix were excluded from further studies because they did not exhibit any growth inhibitory activity against C. albicans in liquid culture, although they showed antifungal activity in the paper disc method. Extracts of the remaining six plants, Acori Gramineri Rhizoma, Angelicae Tenuissimae Radix, Impatientis Semen, Moutan Cortex Radicis, Phellodendri Cortex, and Scutellariae Radix, showed antifungal activity against C. albicans in liquid culture (Fig. 1).
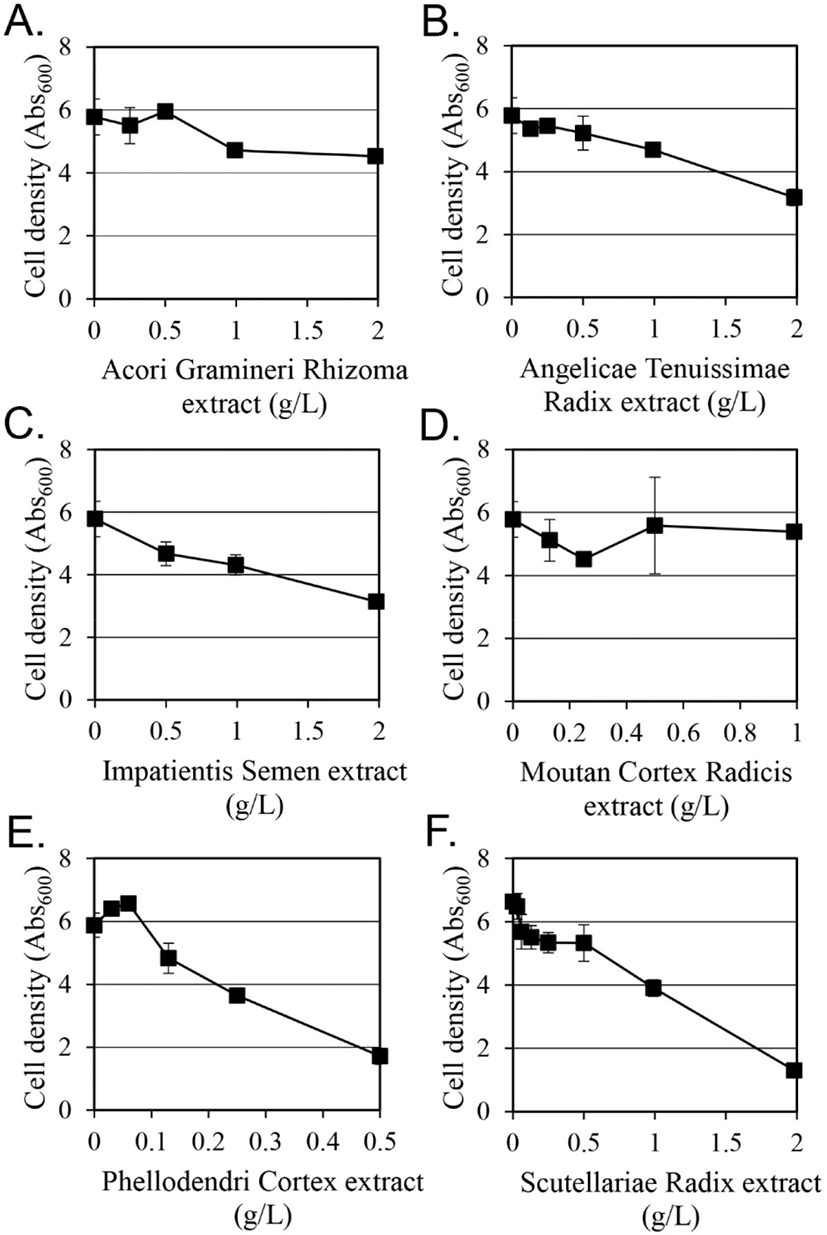
Acori Gramineri Rhizoma extract showed growth inhibitory activity from concentrations of 1 g/L and above. The mycelial growth inhibition of Cladosporium cucumerinum, Colletotrichum orbiculare, Magnaporthe grisea, and Pythium ultimum by β-asrone, one of the main components of Acori Gramineri Rhizoma, have been studied (Lee et al., 2004; Rajput et al., 2013).
Angelicae Tenuissimae Radix and Impatientis Semen extracts showed similar growth inhibitory effects of 45% and 46%, respectively, at 2 g/L.
Moutan Cortex Radicis extract showed growth inhibitory activity of 21% at 0.25 g/L. At higher concentrations, it did not show any further growth inhibitory activity.
Although the size of the inhibitory zone was not large in the paper disc method, Phellodendri Cortex extract showed relatively good antifungal activity in liquid culture. As the concentration increased from 0.13 g/L, the growth inhibitory activity increased. Antifungal activity of water and ethanol extracts of Phellodendri Cortex against C. albicans has been reported in a previous study (Park et al., 1992), and the antifungal activity of berberine and palmatine isolated from Phellodendri Cortex was also reported (Xiao et al., 2015).
The growth inhibition of Scutellariae Radix extracts also increased as the concentration increased at 0.5 g/L or more. It showed an inhibitory effect of 80.4% at 2 g/L. Considering that Scutellariae Radix extracted with ethanol did not show antifungal activity against C. albicans (Cho and Kim, 2001), it is suggested that compounds extracted only with methanol have significant antifungal activity.
Using the 10 selected plant extracts [4 plant extracts from a previous study (Yoon and Kim, 2021) and six plant extracts from Fig. 1], the antifungal activity of 45 combinations of two extracts against C. albicans was evaluated (Fig. 2). To evaluate the synergistic growth inhibitory activity, the concentration of the extract that inhibited only about 20% of the growth was tested. The concentrations of each extract to evaluate the synergistic antifungal activity are shown in Fig. 2. The growth inhibitory activity of 40% or more should be observed to show synergistic growth inhibitory activity. Combinations of extracts showing synergistic growth inhibition of more than 40% were Acori Gramineri Rhizoma and Magnoliae Cortex, Acori Gramineri Rhizoma and Phellodendri Cortex, Angelicae Tenuissimae Radix and Magnoliae Cortex, Magnoliae Cortex and Phellodendri Cortex, Magnoliae Cortex and Syzygii Flos, and Phellodendri Cortex and Syzygii Flos.
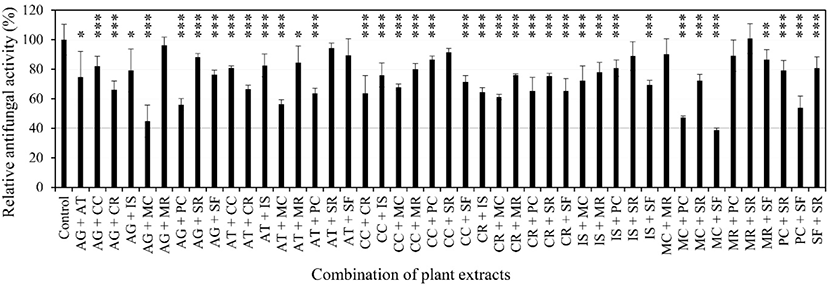
Since the synergistic antifungal activity of the Magnoliae Cortex and Syzygii Flos combination was shown in a previous study (Yoon and Kim, 2021), in this study synergistic antifungal activity was evaluated for the remaining five combinations (Fig. 3).
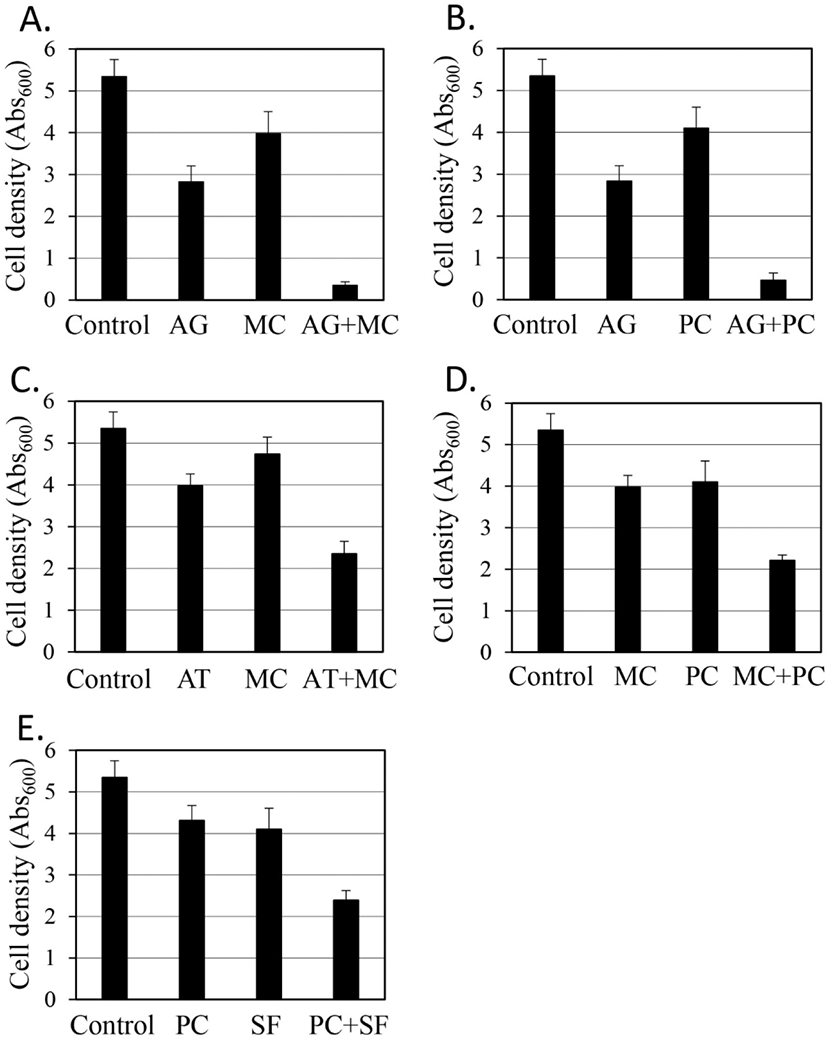
Combination of Acori Gramineri Rhizoma and Magnoliae Cortex extracts (Fig. 3A): The growth inhibitory activity was 47.1% and 25.6% by Acori Gramineri Rhizoma extract (0.99 g/L) and Magnoliae Cortex extract (0.06 g/L), respectively. When the two extracts were mixed, the growth inhibitory activity was 94.5%. A synergistic effect was observed because the inhibitory activity of the mixture was more than double those of the individual extracts.
Combination of Acori Gramineri Rhizoma and Phellodendri Cortex extracts (Fig. 3B): The growth inhibitory activity was 47.1% and 23.3% by Acori Gramineri Rhizoma extract (0.99 g/L) and Phellodendri Cortex extract (0.13 g/L), respectively. When the two extracts were mixed, the growth inhibitory activity was 91.5%. The growth inhibitory activity of the mixture was 3.93 times that of the Phellodendri Cortex extract and 1.94 times that of the Acori Gramineri Rhizoma extract. This showed a partial synergistic effect of the combination of Acori Gramineri Rhizoma and Phellodendri Cortex extracts.
Combination of Angelicae Tenuissimae Radix and Magnoliae Cortex extracts (Fig. 3C): The growth inhibitory activity was 11.4% and 25.6% by Angelicae Tenuissimae Radix extract (0.89 g/L) and Magnoliae Cortex extract (0.06 g/L), respectively. When the two extracts were mixed, the growth inhibitory activity was 56.1%. A synergistic effect was observed because the inhibitory activity of the mixture was more than double those of the individual extracts.
Combination of Magnoliae Cortex and Phellodendri Cortex extracts (Fig. 3D): The growth inhibitory activity was 24.3% and 25.6% by Magnoliae Cortex extract (0.06 g/L) and Phellodendri Cortex extract (0.13 g/L), respectively. When the two extracts were mixed, the growth inhibitory activity was 58.6%. A synergistic effect was observed because the inhibitory activity of the mixture was more than double those of the individual extracts.
Combination of Phellodendri Cortex and Syzygii Flos extracts (Fig. 3E): The growth inhibitory activity was 19.4% and 23.3 by Phellodendri Cortex extract (0.13 g/L) and Syzygii Flos extract (0.12 g/L), respectively. When the two extracts were mixed, the growth inhibitory activity was 55.2%. A synergistic effect was observed because the inhibitory activity of the mixture was more than double those of the individual extracts.
In this study, combinations of plant extracts that simultaneously inhibit the growth of the skin pathogenic fungi C. albicans and M. pachydermatis and the skin bacterium S. aureus were used to synergistically inhibit the growth of C. albicans. The efficacy of these combinations of plant extracts suggests that they could be used for preventing and treating Candida infections, such as stomatitis, vaginitis, and candidemia. Since methanol has safety issues, it is necessary to confirm in additional experiments whether the similar antimicrobial activities are obtained in a safe extraction method using the water or ethanol solvent of the selected 10 plants.
4. CONCLUSIONS
In this study, 10 methanol extracts that simultaneously inhibited C. albicans, M. pachydermatis, and S. aureus, which caused infectious diseases of the skin, were selected from among 240 edible plants. By evaluating all combinations of two extracts, it was identified that five new combinations had partially or fully synergistic antifungal activity. Among the identified combinations, the growth inhibitory activity of 0.06 g/L of Magnoliae Cortex extract with 0.13 g/L of Phellodendri Cortex extract against C. albicans was the strongest with 59% inhibition. The extracts and their combinations in this study provide alternative methods for curing infectious diseases of the skin and developing products for safe skin hygiene.








