Original Article
Phytochemical and Anti-Termite Efficiency Study of Guibourtia tessmanii (harms) J. Léonard (Kévazingo) Bark Extracts from Gabon
Ley-Fleury ELLA NKOGO1,
Christ Stone Arnaud BOPENGA BOPENGA2,†
,
Franck Estimé NGOHANG3,
Line Edwige MENGOME4,
Sophie ABOUGHE ANGONE4,
Prosper EDOU ENGONGA3
Author Information & Copyright ▼
1National School of Water and Forests of Gabon, B.P. 3960, Libreville, Gabon
2Research and Technology Institute (CENAREST/IRT), Gros Bouquet, B.P. 9154, Libreville, Gabon
3Normal Superior School, Department of Physical Sciences, LAPLUS Multidisciplinary Science Laboratory, Hight School Avenue, B.P. 17009, Libreville, Gabon
4Institute of Pharmacopoeia and Traditional Medicine (CENAREST/IPHAMETRA), Sibang, B.P. 1156, Libreville, Gabon
Copyright 2022 The Korean Society of Wood Science & Technology. This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Dec 18, 2021; Revised: Feb 07, 2022; Accepted: Mar 04, 2022
Published Online: Mar 25, 2022
ABSTRACT
This study aimed to explore the biodiversity of chemical compounds found in the bark of Guibourtia tessmannii from Gabon, commonly called Kévazingo, and evaluate their anti-termite activity to determine their potential values as a source of development of anti-termite products that can be valued in the fields of fine chemicals and wood preservation. Extraction of G. tessmannii bark powders was carried out using the cold maceration method with trichloroethylene, acetone, ethanol, and water. Phytochemical screening made it possible to highlight groups of chemical families present in the extracts. Anti-termite activity was tested on the wild termites “Cubitermes sp” of the genus Isoptera. The yield of the extracts were 17.11% for the buttress and 13.42% for the height at 6 m. Phytochemical tests revealed that alkaloids, polyphenols, sterols, tannins, reducing compounds, flavonoids, saponins, and anthraquinones were present in the extracts. Results of anti-termite activity indicated that anti-termite activity varied with the different parts of the bark studied, extraction solvent, and concentration (50/50) and (25/75) of the extracts used. The extracts at 50/50 concentration showed a slightly better anti-termite activity compared to the 25/75 concentration. In addition, the buttress Kévazingo or buttress showed the strongest anti-termite activity for the aqueous extract with a survival rate of 0% after 2 days.
Keywords: extracts; anti-termite activity; Guibourtia tessmannii; phytochemical screening
1. INTRODUCTION
G. tessmannii(harms) J. Léonard commonly called Kévazingo is one of these famous species from Central African countries (Gabon or Cameroon). The abandonment in forests of waste of G. tessmannii in the form of a crown rich in bark and branches of variable diameters, or of slabs, chips, shavings and sawdust in waste reception centers constitutes a considerable financial loss and a risk of losing a deposit biomolecules of interest from this essence widely used in the treatment of several diseases (Obeng, 2011; Raponda-Walker and Sillans, 1961). According to Pr Lee White, Minister of Water and Forests of Gabon, the sustainable cuts of Kévazingo will amount to between 50,000 and 60,000 m3 per year since 2020. It has been observed that in Gabon, a large number of potentially recoverable waste is left in the openings after felling and during shaping in the yard. In addition, the literature reports that 400 million m3 of wood waste is produced in Gabon (Nze Nguema, 2009). These wastes are often thrown away in the form of debris or used by the population as firewood, artisanal charcoal making or sometimes by the boilers of wood processing units. Thus, the issue of recycling tropical wood waste from logging and industrial processing aimed at the sustainable development of the forest economy in the countries of the Congo Basin is the subject of numerous studies. Wood chemicals, among others, offer new perspectives in this area and allow access to new markets. Indeed, bioactive molecules or extractable molecules from renewable resources such as wood are subject to a great deal of research and development in the pharmaceutical, agrifood and cosmetic fields (Isman and Machial, 2006). Previous work carried out on G. tessmanii (Harms) J. Léonard from Gabon has shown that acetone-water, ethanol-water and aqueous extracts have interesting antimicrobial, anti-inflammatory and cytotoxic properties (Sima Obiang et al., 2021). Similarly, some wood species, as is the case with G. tessmanii, naturally resist termite attacks due to their high content of extractable compounds with interesting properties that are part of their natural defense systems (Verma et al., 2009). This type of study in general is more widespread and developed on the extractables of temperate species in industrialized countries. However, tropical woods have higher levels of extractable molecules, which further reinforces the interest in upgrading them (Huang et al., 2009; Mounguengui, 2008; Saha Tchinda, 2015; Zaremski et al., 2009). This valuation of wood represents a major opportunity on the economic level, by the creation of new jobs constituting a factor of growth. In this context, the aim of our work was to determine (i) the extractable content, then (ii) the phytochemical screening and finally (iii) to study the anti-termite activity of these extractives on the wild termites “Cubitermes sp.” of the genus Isoptera from Gabon.
2. MATERIALS and METHODS
2.1. Termites
The handling and harvesting of the colony of wild termites “Cubitermes sp.” of the genus Isoptera was carried out on the day of the experiments so that the withdrawal from their natural habitat had no significant influence on their mortality. For the anti-termite tests, the worker termites were put in a small empty butter pot with the clods of soil and its perforated lid using a heated needle. The termites were immediately brought to the laboratory of the Iphamétra (Pharmacopoeia Institute of Traditional Medicine) in Libreville (Gabon) for testing.
2.2. Plant material
The bark sampling phase was carried out according to a previously established procedure. The mature Kévazingo bark was collected using a machete on April 8, 2020. The collection of Kévazingo bark in Kango, in the Estuary province (N 00°02’44.5” and E 010°17’23.7”) of size 10 to 15 cm was carried out on two heights on the same tree: at the level of the buttress and six meters from the ground. Because in general, the content of secondary metabolites, also called extractable, decreases with height in the tree (Caron et al., 2013). The bark was cut into small pieces using a pair of scissors. They were then sent to the Multidisciplinary Science Laboratory (LaPluS) of the Ecole Normale Supérieure de Libreville where they were dried in the open air for three weeks.
2.3. Extraction
The extraction of the chemical elements began with the fragmentation and grinding of the bark. The bark was first cut with scissors to facilitate crushing; the fragments obtained were then introduced in small quantities into a Retsch-type mill, with Iphametra. This is necessary to obtain a suitable grain size with a fraction between 1 and 2 mm, thus corresponding to the requirements of ASTM No. 1105 (1996) in terms of grain size to quantify the rate of wood extract. Finally, the powder collected was sieved and stored in the dark in glass jars, closed until the time of chemical tests. The chemical tests began with the determination of the extract levels. We opted for the successive extraction by cold maceration of the bark powders using solvents of increasing polarities in the order of Trichloroethylene, Acetone, ethanol, and Distilled water. The bark powder was placed in a 1/10 ratio (10 g of dry matter in 100 mL of solvent) in a 250 mL glass Erlenmeyer flask, closed with a rubber stopper covered with aluminum foil. The aluminum thus prevents the solvent from coming into contact with the rubber, which prevents possible contamination. In addition, the Erlenmeyer flasks were covered with aluminum foil to prevent degradation of photosensitive molecules. The mixtures were subsequent placed under stirring on a stirrer (of the PIERRON type) for 24 h. After 24 h, the mixtures were separated by vacuum filtration through a Whatman filter in a Buchner type funnel. With regard to the extraction in aqueous medium, the water is evaporated by lyophilization. The extract solutions were then evaporated in flasks (weighed beforehand) using a rotary evaporator, in a 40°C. bath and were then placed in a desiccator until constant mass and weighed again. The extracts are then stored in the refrigerator in closed bottles and covered with aluminum foil for the next tests. We can thus calculate the percentage of extracts relative to the initial mass of bark powder using the following Equation (1):
Where:
R: yield of extracts in%
Mext: mass of the extract after evaporation in grams
Mech : anhydrous mass of the bark powder sample in grams.
2.4. Phytochemical screening
The reagents used to carry out the phytochemical screening of the extracts were prepared and used according to the protocols described by (Akinjogunla et al., 2010; Badiaga, 2012; Houghton and Raman, 1998). All the different tests were carried out in triplicate.
2.4.1. Alkaloids analysis
For alkaloids, 10 mL of extract was placed in a test tube and then a few drops of Dragendorff’s reagent solution were added. The appearance of a red-orange colored precipitate indicated the presence of the alkaloids (N’Guessan, 2009).
2.4.2. Polyphenols analysis
For polyphenols, 2 mL of extract was placed in a test tube and then a few drops of the 2% ethanolic ferric chloride solution were added. The appearance of a blue-blackish color indicates the presence of polyphenols (N’Guessan, 2009).
2.4.3. Sterols and triterpenes analysis
For the detection of sterols and terpenes, 20 mg of extracts, 3 mL of chloroform, 10 drops of acetic anhydride and 2 drops of concentrated sulfuric acid were introduced into a test tube. The appearance of a purple ring, turning blue and then green, indicated the presence of sterols and terpenes (Bopenga Bopenga et al., 2020a, 2020b).
2.4.4. Tannins analysis
The presence of tannins was demonstrated by adding to 1 mL of extract, 1 mL of distilled water and 1 to 2 drops of FeCl3 solution [iron perchloride or iron (III) chloride] diluted to 1%. The appearance of a dark green color indicates the presence of tannins (Badiaga, 2012).
2.4.5. Reducing compounds analysis
For the reducing compounds introduced, 2 mL of the extract in a test tube, then 2 mL of Fehling’s liquor is added. The whole was then put in a boiling water bath for 8 minutes. The appearance of a brick red precipitate indicated the presence of reducing compounds (Bentabet, 2015).
2.4.6. Flavonoids analysis
For flavonoids, 1 mL of extract was placed in a test tube, then 1 mL of hydrochloric acid, 1 mL of isoamyl alcohol were added and then some magnesium shavings were added. The appearance of a pinkish-orange color indicates the presence of flavonoids (Harbone and Williams, 2000).
2.4.7. Saponins analysis
The saponins were identified by introducing 10 mL of each extract into a test tube and then vigorously shaking with a vortex for 15 seconds. The tube is left to stand for 15 minutes. The appearance of persistent foam indicates the presence of saponins (N’Guessan, 2009).
2.4.8. Anthraquinones analysis
For anthraquinones, to 2 mL of each extract is added 1 mL of 10% NH4OH (basic aqueous ammonia solution). After shaking, the appearance of a purple color indicates a positive test (Oloyede, 2005).
2.5. Anti-termite activity
The protocol described for the anti-termite tests was inspired by those described by (Bédounguidzi et al., 2020; Bopenga Bopenga et al., 2020a, 2020b). Only the acetone, ethanolic and aqueous extracts were tested. Two concentrations were tested against wild termites for the screening tests, mass ratios in percentage of (50:50) and (25:75) (extract: extraction solvent). 70 μL of solutions were impregnated on Whatman filter papers before being exposed to termites. The papers impregnated with the various solutions to be tested were dried in the open air, 27°C/75% relative humidity (RH) for 2 hours. The tests were carried out in Petri dishes (9 cm in diameter) where 15 g of wet sand (1 volume of water for 4 volumes of sand) were placed at the periphery. The Whatman extracts soaked papers were placed on a plastic rack in the middle of the petri dish. Then 20 wild worker termites were added to each test device without any possibility of feeding to check termite survival. For each concentration tested, we prepared three Petri dishes with extract and three Petri dishes with extraction solvent without extract which were used as controls during the test. Petri dishes were stored in the dark at 27°C, 75% RH for 12 days. At the end, the samples were cleaned and air dried. These devices were monitored regularly throughout the trial. The test is then stopped when all of the termites in the boxes tested have died. Mortality and daily survival rates were respectively calculated according to Equation (2) and deduced according to Equation (3). The results of the screening tests were presented in the form of curves of survival rates as a function of day and time of incubation in order to facilitate interpretations.
2.6. Statistical analysis
XLSTAT version 2019 (ADDINSOFT SARL) was used, and the results were known as the main values representing the mean of the repetitions ± SD. The. The values are statistically significant at p < 0.05.
3. RESULTS and DISCUSSION
3.1. The extracts contents
In general, the results obtained in this study concerning the extraction yields indicate that the rate of extractables vary from one solvent to another, in the two samples of Kévazingo bark, the base and therefore the buttress up to 6 m. Acetone extraction rates are higher in samples E1KC, E2KC (9.92% and 6.82% respectively). The rates of extracts obtained with trichlorethylene and with water are the lowest regardless of the type of sample. Indeed, for trichlorethylene, the first solvent used during the successive extraction is the least polar solvent and therefore dissolves the phenolic compounds less. Non-polar solvents mainly extracted from nonpolar substances (oils, fats, terpenes) while polar solvents dissolve polar compounds such as polyphenols. The successive extraction, which combines non-polar and polar solvents, allows the extractables to be partitioned into different fractions, facilitating subsequent analyzes and the sum of the extracts with each solvent gives an idea of the overall extract content of the bark. The overall extract rates of the different parts studied vary from 17.11% to 13.42% respectively for the buttress and the height at 6 m (Table 1). The results showed that the content of total extracts varies depending on the diameter and height of the trees (the level of extractables decreases with height and increases with diameter). These results do not differ from most of those obtained by other authors. Indeed, Rather et al. (2017) obtained significantly higher extraction yields for Eucalyptus (Eucalyptus terepticornis) in the wood at the base of the trunk compared to the rest of the tree. In addition, numerous studies carried out on several tropical species have shown very variable extract rates, sometimes with high levels of 20% to 22% for certain woods (Cheumani, 2009; Huang et al., 2009; Mburu et al., 2007; Neya et al., 2004; Sirmah, 2009).
Table 1.
Extracts contents obtained by maceration
| Extracts contents (average of three tests ± SD) |
| Solvant |
Extracts contents (%)1) |
| E1KC |
E2KC |
| Trichloroéthylène |
0.79 ± 0.11 |
1.0 ± 0.11 |
| Acetone |
9.92 ± 0.14 |
6.82 ± 0.13 |
| Ethanol |
6.23 ± 0.07 |
5.39 ± 0.12 |
| Water |
0.17 ± 0.07 |
0.21 ± 0.01 |
| Total |
17.11 |
13.42 |
Download Excel Table
3.2. Phytochemical screening
Overall, no significant difference was observed in the results of the phytochemical screening on the E1KC and E2KC samples. It appears from Tables 2 and 3 that the Alkaloids, Polyphenols, Tannins, Reducing compounds, Flavonoids and Anthraquinones were identified in the acetone, ethanol, and aqueous extracts of the bark of Guibourtea tessmanii. In addition, Polyphenols were also identified in the trichloroethylene extracts of the bark of the E1KC sample. Saponins were identified only in aqueous extracts. These compounds are identified in the literature for their antioxidant, antifungal, anti-termite, antibacterial, antimicrobial properties (Bopenga Bopenga et al., 2020a, 2020b; Jeong et al., 2017; Kim et al., 2018; Min et al., 2019; Oliveira et al., 2010; Ohmura, 2000; Wu and Lin, 2001). Sterols and triterpenes identified only in trichloroethylene extracts. Terpene compounds are known in the literature (Andréa et al., 2010; Becker et al., 2005; Bédounguindzi et al., 2020; Bläske et al., 2003; Escalante et al., 2002; Lajide et al., 1995; Park and Shin, 2005; Watanabe et al., 2005; Viegas, 2003) for their antifungal and anti-termite activities. Our phytochemical screening results are in line with those obtained by Sima Obiang et al. (2018) which demonstrated the presence of saponines, tannins, phenols and flavonoids, alkaloids, reducing compounds, anthraquinone, sterols in the bark of barks of Coula edulis Baill, Pseudospondias longifolia Engl and Carapa klaineana Pierre from Gabon. The presence of all classes of compounds in tropical timber has been reported in the literature (Kilic and Niemz, 2012; Masendra et al., 2019), when studying extractables from some tropical species, found lipophilic compounds, mainly fatty acids and hydrophilic compounds (phenolic acids, flavonoids, sterols, stilbenes, and lignans).
Table 2.
Phytochemical screening (E1KC)
| Active compounds |
Extraction solvent1) |
| Trichloroethylene |
Acetone |
Ethanol |
Water |
| Alkaloids |
+ |
+ |
+ |
+ |
| Polyphenols |
+ |
+ |
+ |
+ |
| Sterols and triterpenes |
+ |
− |
− |
− |
| Tannins |
− |
+ |
+ |
+ |
| Reducing compounds |
− |
+ |
+ |
− |
| Flavonoids |
− |
+ |
+ |
− |
| Saponins |
− |
− |
− |
+ |
| Anthraquinones |
− |
+ |
+ |
+ |
Download Excel Table
Table 3.
Phytochemical screening (E2KC)
| Active compounds |
Extraction solvent1) |
| Trichloroethylene |
Acetone |
Ethanol |
Water |
| Alkaloids |
+ |
+ |
+ |
+ |
| Polyphenols |
+ |
+ |
+ |
+ |
| Sterols and triterpenes |
+ |
− |
+ |
− |
| Tannins |
− |
+ |
+ |
+ |
| Reducing compounds |
− |
+ |
+ |
+ |
| Flavonoids |
− |
+ |
+ |
− |
| Saponins |
− |
− |
− |
+ |
| Anthraquinones |
− |
+ |
+ |
+ |
Download Excel Table
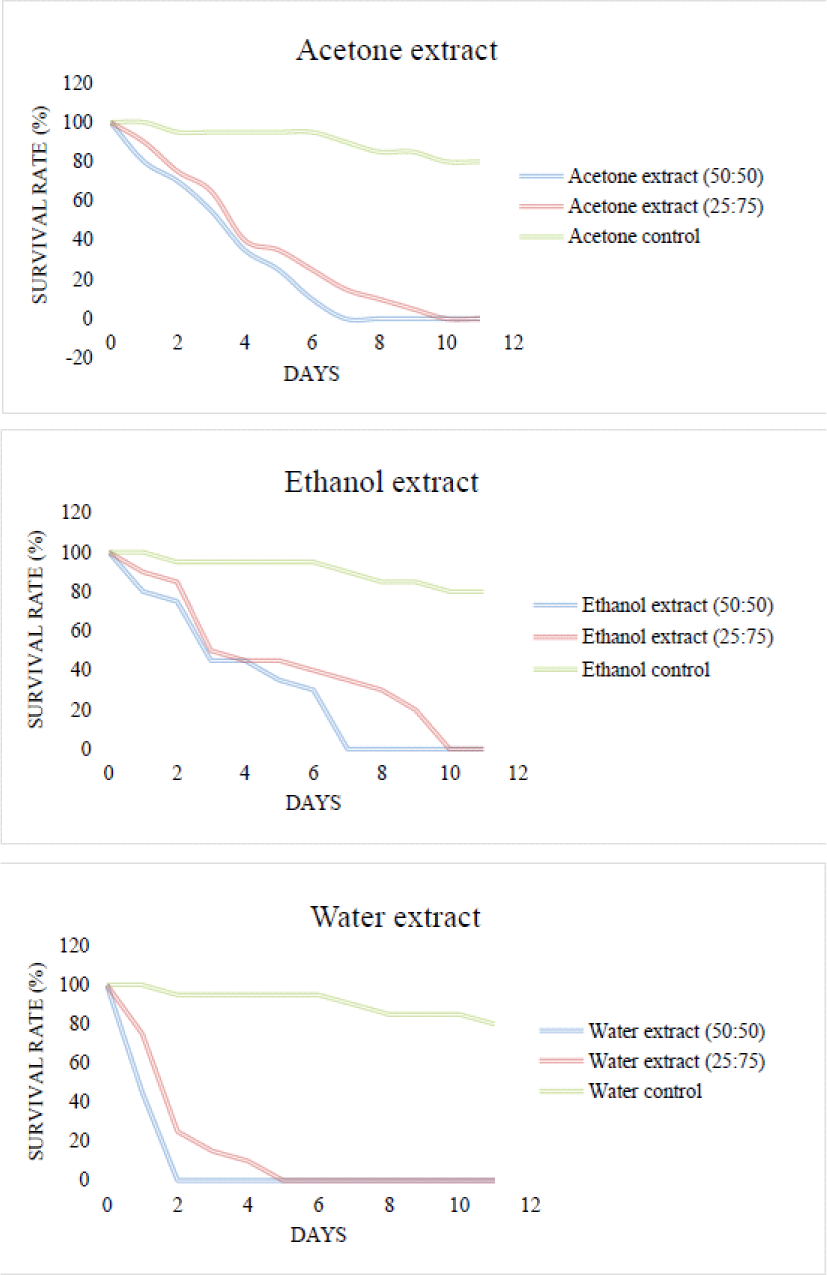
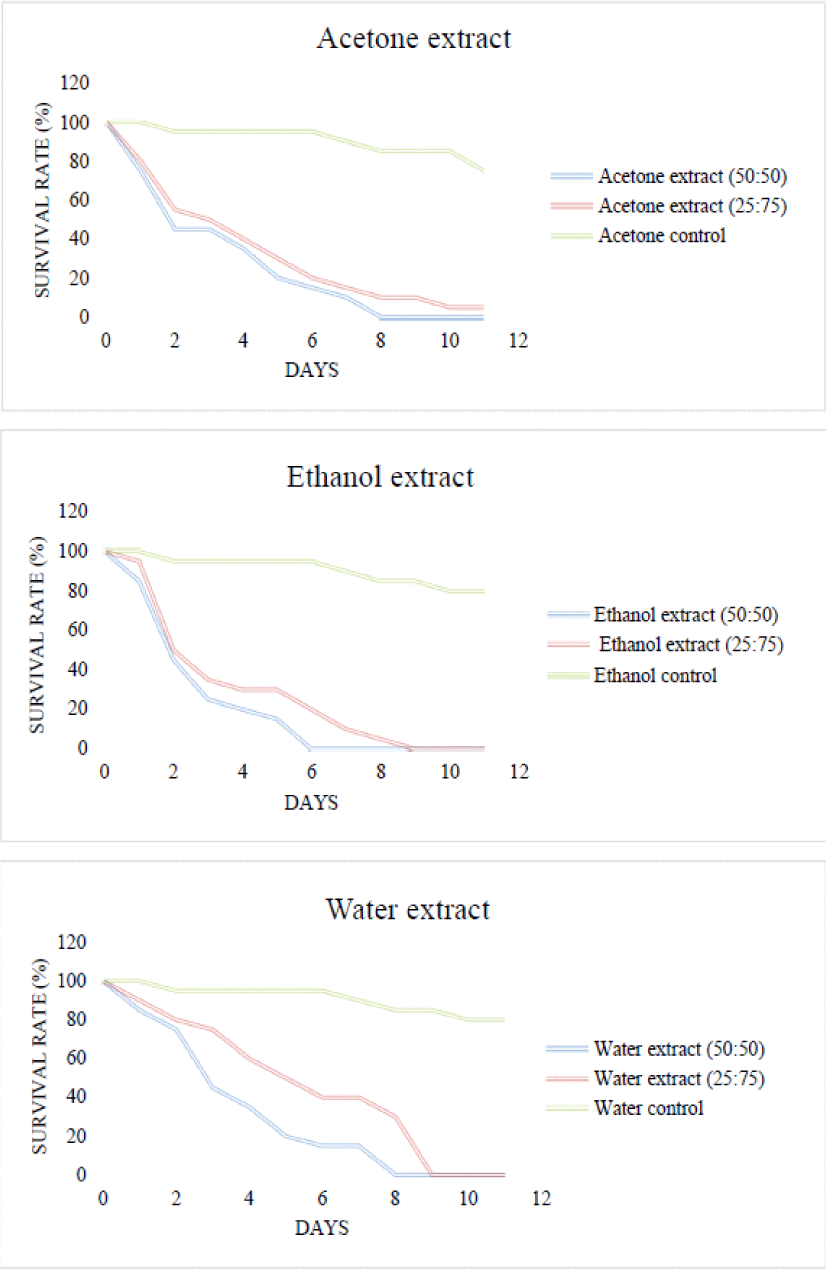
3.3. Anti-termite activity
The results obtained (Fig. 1 and 2) indicate that the anti-termite activity varies with the different parts of the bark studied (heights), the extraction solvent and the concentration of the extracts used as often reported in the literature (Andréa et al., 2010). Overall, the extracts at concentrations (50/50) exhibit slightly better anti-termite activity compared to (25/75). The strongest anti-termite activities were recorded with the aqueous extract of the E1KC sample with a survival rate of 0% after 2 days, explaining the concentration of the extracts at this level. Diluting the solutions to a concentration of (25/75) increases the survival rate. These results demonstrate that the control paper impregnated only with water or acetone has no effect on the behavior of termites as shown by the survival rates (termite survival > 12 days with 80%) at the end of the trial. Basically all papers impregnated with extracts showed a protective effect of whatman paper, therefore strong resistance against termites. For each of these extracts, all the termites died before the end of the test. However, the use of less concentrated solutions still resulted in a 0% survival rate, hence anti-termite activity. Previous analyzes, in particular phytochemical screening, have made it possible to identify secondary metabolites such as polyphenols, tannins, flavonoids and anthraquinones, making it possible to explain the good anti-termite activity of these extracts (Jung et al., 2017; Morina et al., 2020). The results are in agreement with the literature which explains that tannins are known to be strongly astringent (Lee et al., 2020; Min et al., 2017; Monteiro et al., 2005; Mun and Darrel, 2017). Ayres et al. (1997) verified that the high mortality of insects treated with condensed and hydrolyzable tannins appears to be due to the toxic properties of these compounds and not to inhibition of digestion. Phytochemical screening revealed the presence of flavonoids in these extracts which are potential termite control agents (Hadi et al., 2020; Manurung et al., 2019; Nam et al., 2018; Simmonds, 2001). Other previous studies have also reported their anti-termite activity (Arsyad et al., 2020; Chen et al., 2004; Morimoto et al., 2006) and have shown that flavonoids such as catechin interact with the ecdysone receptor of termites (Oberdörster et al., 2001). Due to the ability of flavonoids to bind to ecdysone receptors, flavonoids can affect other biological systems in termites (Boué and Raina, 2003). Ragon et al. (2008) hypothesized that termites detected and avoided woods containing the extracts rich in antioxidant molecules because they could interfere with the digestion of lignocellulose by termite symbionts. Anthraquinones are very present in the ethanolic and aqueous extracts for the sample at 6 m and very abundant in the acetone, ethanolic and aqueous extracts for the buttress or certain quinones such as 2-methylanthraquinone are repellent against termites, others such as 7-methyljuglone and its derivatives have anti-termite activities (Arsyad et al., 2019; Hadi et al., 2018; Leyva et al., 1998; Niamké et al., 2012; Thévenon et al., 2001). These results explain the importance of phenolic compounds in the resistance of wood to termites.
Fig. 1.
Kinetics of the daily survival rate of termites according to the concentrations of the different extracts of the E1KC sample. E1KC: sample 1 Kévazingo from Kango collected at the buttress.
Download Original Figure
Fig. 2.
Kinetics of the daily survival rate of termites according to the concentrations of the different extracts of the E2KC sample. E2KC: sample 2 Kévazingo from Kango collected at 6 m.
Download Original Figure
4. CONCLUSION
This study looked at the variability in the height of the extract rates from the bark of Kévazingo. On the other hand, the elucidation of the families of chemical compounds likely to be active by phytochemical tests and the evaluation of their anti-termite properties. As a result of this work, we have achieved a large number of results. Thus, it turns out that the results showed extractable levels which varied from the buttress to the height of 6 m and according to the type of solvent used. The total extractable level of the samples studied is E2KC (13.42%) and E1KC (17.11%). Photochemical screening revealed the presence of different groups of molecules such as polyphenols, alkaloids, sterols, tannins, reducing compounds, flavonoids, saponins, anthraquinones. The results obtained indicated that the anti-termite activity varies with the parts of the bark studied (heights), the extraction solvent and the concentration of the extracts used as often reported in the literature. In addition, the extracts at concentrations (50/50) showed slightly better anti-termite activity compared to (25/75) and buttress Kévazingo or buttress showed the highest anti-termite activity for the aqueous extract with a higher level of anti-termite activity. 0% survival rate after 2 days.
ACKNOWLEDGMENT
The authors would like to thank the following institutions and laboratories: CENAREST/ IPHAMÉTRA, CENAREST/IRT, ENEF, LaReVaBois, ENS and ENSET in Libreville, Gabon.
REFERENCES
Akinjogunla, O.J., Yah, C.S., Eghafona, N.O., Ogbemudia, F.O. 2010. Antibacterial activity of leave extracts of
Nymphaea lotus (Nymphaeaceae) on
Methicillin resistant
Staphylococcus aureus (MRSA) and
Vancomycin resistant
Staphylococcus aureus (VRSA) isolated from clinical samples. Annals of Biological Research 1(2): 174-184.

Andréa, L.B.D.S., Maranhão, A.C., Santos, J.C., Cunha, F.M., Conceição, G.M., Bieber, L.W., Nascimento, M.S. 2010. Antitermitic activity of extractives from three Brazilian hardwoods against
Nasutitermes corniger. International Biodeterioration & Biodegradation 64(1): 7-12.


Arsyad, W.O.M., Basri, E., Hendra, D., Trisatya, D.R. 2019. Termite resistance of impregnated Jabon wood (
Anthocephalus cadamba Miq.) with combined impregnant agents. Journal of the Korean Wood Science and Technology 47(4): 451-458.


Arsyad, W.O.M., Efiyanti, L., Trisatya, D.R. 2020. Termiticidal activity and chemical components of bamboo vinegar against subterranean termites under different pyrolysis temperatures. Journal of the Korean Wood Science and Technology 48(5): 641-650.


Ayres, M.P., Clausen, T.P., MacLean S.F. Jr., Redman, A.M., Reichardt, P.B. 1997. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 78(6): 1696-1712.


Badiaga, M. 2011. Ethnobotanical, phytochemical and biological study of
Nauclea latifolia Smith, an African medicinal plant collected in Mali. Ph.D. Thesis, University of Bamako, Mali. pp. 30-32.

Becker, H., Scher, J.M., Speakman, J.B., Zapp, J. 2005. Bio-activity guided isolation of antimicrobial compounds from
Lythrum salicaria. Fitoterapia, 76(6): 580-584.



Bédounguindzi, W.F., Candelier, K., Edou Engonga, P., Dumarçay, S., Thévenon, M.F., Gérardin, P. 2020. Anti-termite and anti-fungal bio-sourced wood preservation ingredients from
Dacryodes edulis (G. Don) H.J. Lam resin. Holzforschung 74(8): 745-753.


Bentabet, L.N. 2015. Phytochemical study and evaluation of the activities of two plants
Fredolia aretioides and
Echium vulgare from western Algeria. Ph.D. Thesis, Aboubekr Belkaïd Tlemcen University, Algeria, p. 22.

Bläske, V.U., Hertel, H., Forschler, B.T. 2003. Repellent effects of isoborneol on subterranean termites (
Isoptera: Rhinotermitidae) in soils of different composition. Journal of Economic Entomology 96(4): 1267-1274.



Bopenga Bopenga, C.S.A., Dumarçay, S., Edou Engonga, P., Gérardin, P. 2020a. Relationships between chemical composition and decay durability of
Coula edulis Baill as an alternative wood species in Gabon. Wood Science and Technology 54(2): 329-348.


Bopenga Bopenga, C.S.A., Meyo Degboevi, H., Candelier, K., Edou Engonga, P., Dumarçay, S., Thévenon, M.F., Gérardin Charbonnier, C., Gérardin, P. 2020b. Characterization of extracts from the bark of the Gabon hazel tree (
Coula edulis Baill) for antioxidant, antifungal and anti-termite products. Journal of Renewable Materials 9(1): 17-33.


Boué, S.M., Raina, A.K. 2003. Effects of plant flavonoids on fecundity, survival, and feeding of the
Formosan subterranean termite. Journal of Chemical Ecology 29(11): 2575-2584.



Caron, A., Altaner, C.M., Gardiner, B., Jarvis, M.C. 2013. Distribution of extractives in Sitka spruce (
Picea sitchensis) grown in the northern UK. European Journal of Wood and Wood Products 71(6): 697-704.


Cheumani, Y.A.M. 2009. Étude de la microstructure des composites bois/ciment par relaxométrie RMN du proton. Ph.D. Thesis, Université de Bordeaux 1, France.

Escalante, A.M., Santecchia, C.B., López, S.N., Gattuso, M.A., Ravelo, A.G., Monache, F.D., Sierra, M.G., Zacchino, S.A. 2002. Isolation of antifungal saponins from
Phytolacca tetramera, an Argentinean species in critic risk. Journal of Ethnopharmacology 82(1): 29-34.


Hadi, Y.S., Massijaya, M.Y., Abdillah, I.B., Pari, G., Wa Arsyad, W.O.M. 2020. Color change and resistance to subterranean termite attack of Mangium (
Acacia mangium) and Sengon (
Falcataria moluccana) smoked wood. Journal of the Korean Wood Science and Technology 48(1): 1-11.


Hadi, Y.S., Massijaya, M.Y., Zaini, L.H., Abdillah, I.B., Arsyad, W.O.M. 2018. Resistance of methyl methacrylate-impregnated wood to subterranean termite attack. Journal of the Korean Wood Science and Technology 46(6): 748-755.


Harborne, J.B., Williams, C.A. 2000. Advances in flavonoid research since 1992. Phytochemistry 55(6): 481-504.


Houghton, P.J., Raman, A. 1998. Laboratory Handbook for the Fractionation of Natural Extracts. Springer, London, UK.


Huang, Z., Hashida, K., Makino, R., Kawamura, F., Shimizu, K., Kondo, R., Ohara, S. 2009. Evaluation of biological activities of extracts from 22 African tropical wood species. Journal of Wood Science 55(3): 225-229.


Iacobellis, N.S., Lo Cantore, P., Capasso, F., Senatore, F. 2005. Antibacterial activity of
Cuminum cyminum L. and
Carum carvi L. essential oils. Journal of Agricultural and Food Chemistry 53(1): 57-61.



Isman, M.B., Machial, C.M. 2006. Pesticides based on Plant Essential Oils: From Traditional Practice to Commercialization. In: Naturally Occurring Bioactive Compounds, Ed. by Rai, M. and Carpinella, M.C., Elsevier, Amsterdam, Netherland.


Jeong, M.J., Yang, J., Choi, W.S., Kim, J.W., Kim, S.J., Park, M.J. 2017. Chemical compositions and antioxidant activities of essential oil extracted from
Neolitsea aciculata (Blume) Koidz leaves. Journal of the Korean Wood Science and Technology 45(1): 96-106.


Jung, J.Y., Ha, S.Y., Yang, J.K. 2017. Response surface optimization of phenolic compounds extraction from steam exploded oak wood (
Quercus mongolica). Journal of the Korean Wood Science and Technology 45(6): 809-827.


Kilic, A., Niemz, P. 2012. Extractives in some tropical woods. European Journal of Wood and Wood Products 70(1-3): 79-83.


Kim, J.W., Um, M., Lee, J.W. 2018. Antioxidant activities of hot water extracts from different parts of rugosa rose (
Rosa rugosa Thunb.). Journal of the Korean Wood Science and Technology 46(1): 38-47.


Lajide, L., Escoubas, P., Mizutani, J. 1995. Termite antifeedant activity in
Xylopia aethiopica. Phytochemistry 40(4): 1105-1112.


Lee, M., Jeong, S.H., Mun, S.P. 2020. Conditions for the extraction of polyphenols from radiata pine (
Pinus radiata) bark for bio-foam preparation. Journal of the Korean Wood Science and Technology 48(6): 861-868.


Leyva, A., Dimmel, D.R., Pullman, G.S. 1998. Teak extract as a catalyst for the pulping of loblolly pine. Tappi Journal 81(5): 237-240.

Manurung, H., Sari, R.K., Syafii, W., Cahyaningsih, U., Ekasari, W. 2019. Antimalarial activity and phytochemical profile of ethanolic and aqueous extracts of bidara laut (
Strychnos ligustrina Blum) wood. Journal of the Korean Wood Science and Technology 47(5): 587-596.


Masendra, Ashitani, T., Takahashi, K., Susanto, M., Lukmandaru, G. 2019. Hydrophilic extracts of the bark from six
Pinus species. Journal of the Korean Wood Science and Technology 47(1): 80-89.


Mburu, F., Dumarçay, S., Gérardin, P. 2007. Evidence of fungicidal and termicidal properties of
Prunus africana heartwood extractives. Holzforschung 61(3): 323-325.


Min, H.J., Kim, E.J., Shinn, S., Bae, YS. 2019. Antidiabetic activities of Korean red pine (
Pinus densiflora) inner bark extracts. Journal of the Korean Wood Science and Technology 47(4): 498-508.


Min, H.J., Kim, Y.K., Bae, Y.S. 2017. Evaluation of biological activity on hawthorn tree (
Crataegus pinnatifida) extracts. Journal of the Korean Wood Science and Technology 45(3): 317-326.


Monteiro, J.M., Lins Neto, E.M.F., Amorim, E.L.C., Araújo, E.L., Albuquerque, U.P. 2005. Tannins: From chemistry to ecology. Química Nova 28: 892-896.


Morimoto, M., Fukumoto, H., Hiratani, M., Chavasiri, W., Komai, K. 2006. Insect antifeedants, pterocarpans and pterocarpol, in heartwood of
Pterocarpus macrocarpus Kruz. Bioscience Biotechnology and Biochemistry 70(8): 1864-1868.



Morina, A.D.F.A., Romayasa, A., Kusnanda, A.J., Avidlyandi, A., Yudha, S.S., Banon, C., Gustian, I. 2020. Chemical components, antitermite and antifungal activities of
Cinnamomum parthenoxylon wood vinegar. Journal of the Korean Wood Science and Technology 48(1): 107-116.


Mounguengui, S., Saha Tchinda, J.B., Kor Ndikontar, M., Dumarçay, S., Attéké, C., Perrin, D., Gelhaye, E., Gérardin, P. 2016. Total phenolic and lignin contents, phytochemical screening, antioxidant and fungal inhibition properties of the heartwood extractives of ten Congo Basin tree species. Annals of Forest Science 73(2): 287-296.


Mun S.P., Darrel, D.N. 2017. Effect of proanthocyanidin-rich extracts from
Pinus radiata bark on termite feeding deterrence. Journal of the Korean Wood Science and Technology 45(6): 720-727.


Nam, J.B., Oh, G.H., Yang, S.M., Lee, S.E., Kang, S.G. 2018. Evaluation of antioxidant activities of water extract from microwave torrefied oak wood. Journal of the Korean Wood Science and Technology 46(2): 178-188.


N’guessan, J.D., Zirihi, G.N., Kra, A.K.M., Kouakou, K., Djaman, A.J., Guede-guina, F. 2007. Free radical scavenging activity, flavonoid and phenolic contents of selected Ivoirian plants. International Journal of Natural and Applied Sciences 3(4): 425-429.


Neya, B., Hakkou, M., Pétrissans, M., Gérardin, P. 2004. On the durability of
Burkea africana heartwood: Evidence of biocidal and hydrophobic properties responsible for durability. Annals of Forest Science 61(3): 277-282.


Niamké, F.B., Amusant, N., Stien, D., Chaix, G., Lozano, Y., Kadio, A.A., Lemenager, N., Goh, D., Adima, A.A., Kati-Coulibaly, S., Jay-Allemand, C. 2012. 4’,5’-Dihydroxy-epiisocatalponol, a new naphthoquinone from
Tectona grandis L. f. heartwood, and fungicidal activity. International Biodeterioration & Biodegradation 74: 93-98.


Obeng, E.A. 2011.
Guibourtia tessmannii (Harms) J. Leonard. In: PROTA: Plant Resources of Tropical Africa, Ed. by Lemmens, R.H.M.J., Louppe, D. and Oteng-Amoako, A.A. PROTA Foundation, Wageningen, Netherlands.

Oberdörster, E., Clay, M.A., Cottam, D.M., Wilmot, F.A., McLachlan, J.A., Milner, M.J. 2001. Common phytochemicals are ecdysteroid agonists and antagonists: A possible evolutionary link between vertebrate and invertebrate steroid hormones. The Journal of Steroid Biochemistry and Molecular Biology 77(4-5): 229-238.


Ohmura, W., Doi, S., Aoyama, M., Ohara, S. 2000. Antifeedant activity of flavonoids and related compounds against the subterranean termite
Coptotermes formosanus Shiraki. Journal of Wood Science 46(2): 149-153.


Oliveira, L.S., Santana, A.L.B.D., Maranhão, C.A., de Miranda, R.C.M., Galvão de Lima, V.L.A. 2010. Natural resistance of five woods to
Phanerochaete chrysosporium degradation. International Biodeterioration & Biodegradation 64(8): 711-715.


Oloyede, O.I. 2005. Chemical profile of unripe pulp of
Carica papaya. Pakistan Journal of Nutrition 4(6): 379-381.


Park, I.K., Shin, S.C. 2005. Fumigant activity of plant essential oils and components from garlic (
Allium sativum) and clove bud (
Eugenia caryophyllata) oils against the Japanese termite (
Reticulitermes speratus Kolbe). Journal of Agricultural and Food Chemistry 53(11): 4388-4392.



Ragon, K.W., Nicholas, D.D., Schultz, T.P. 2008. Termite-resistant heartwood: The effect of the non-biocidal antioxidant properties of the extractives (
Isoptera: Rhinotermitidae). Sociobiology 52(1): 47-54.

Raponda-Walker, A., Sillans, R. 1961. The Useful Plants of Gabon: Inventory and Concordance Test of the Vernacular and Scientific Names of Spontaneous and Introduced Plants. In: Description of species, properties, uses, Ed. by Lechevalier, P., Raponda Walker Foundation, Paris, France.

Rather, S.A., Sharma, K.R., Nabi, S., Heena, G. 2017. Effect of sampling height and spacing on wood extractives in
Eucalyptus tereticornis Smith. Asian Journal of Multidisciplinary Studies 5(4).

Saha Tchinda, J.B. 2015. Characterization and valuation of extractable substances from five major cameroonian species in the wood industry: Ayous, Moabi, Movingui, Padouk and Tali. Ph.D. Thesis, University of Yaoundé I, Cameroon.

Sima Obiang, C., Ngoua Meye Misso, R.L., Ndong Atome, G.R., Obame, R.B.M., Ondo, J.P., Engonga, L.C.O., Emvo, E.N. 2021. Antimicrobial, antioxidant, anti-inflammatory and cytotoxic study of extracts of
Guibourtia tessmanii (harms) J. Léonard from Gabon. Clinical Phytoscience: International Journal of Phytomedicine and Phytotherapy 7(1): 45.


Sima Obiang, C., Ondo, J.P., Ndong Atome, G.R., Obame Engonga, L.C., Djoba Siawaya, J.F., Nsi Emvo, E. 2016. Phytochemical screening, antioxidant and antimicrobial potential of stem barks of
Coula edulis Baill.
Pseudospondias longifolia Engl. and
Carapa klaineana Pierre. from Gabon. Asian Pacific Journal of Tropical Disease 6(7): 557-563.


Simmonds, M.S.J. 2001. Importance of flavonoids in insect–plant interactions: Feeding and oviposition. Phytochemistry 56(3): 245-252.


Sirmah, P., Dumarçay, S., Masson, E., Gérardin, P. 2009. Unusual amount of (−)-mesquitol from the heartwood of
Prosopis juliflora. Natural Product Research 23: 183-189.



Thévenon, M.F., Roussel, C., Haluk, J.P. 2001. Possible durability transfer from durable to non durable wood species: The case study of teak wood. In: Stockholm, Sweden, The International Research Group on Wood Preservation, 32nd Annual Meeting, IRG/WP 01-10392.

Verma, M., Sharma, S., Prasad, R. 2009. Biological alternatives for termite control: A review. International Biodeterioration & Biodegradation 63(8): 959-972.


Viegas C. Jr., 2003. Terpenos com atividade inseticida:
Uma alternativa para o controle quimico de insetos. Quimica Nova 26(3): 390-400.


Watanabe, Y., Mihara, R., Mitsunaga, T., Yoshimura, T. 2005. Termite repellent sesquiterpenoids from
Callitris glaucophylla heartwood. Journal of Wood Science 51(5): 514-519.


Wu, T.S., Lin, F.W. 2001. Alkaloids of the wood of
Cryptocarya chinensis. Journal of Natural Products 64(11): 1404-1407.



Yam, M.F., Ang, L.F., Ameer, O.Z., Salman, I.M., Aziz, H.A., Asmawi, M.Z. 2009. Anti-inflammatory and analgesic effects of
Elephantopus tomentosus ethanolic extract. Journal of Acupuncture and Meridian Studies 2(4): 280-287.


Zaremski, A., Fouquet, D., Louppe, D. 2009. Les Termites Dans le Monde. Quae: Versailles, France.