1. INTRODUCTION
The camellia is valued not only for its aesthetic contribution as an ornamental tree or shrub but also for its economic importance as it provides the beverage, tea, and edible oil in some countries, notably China (Rolfe, 1992). Apart from its use for ornamental purposes and production of tea, camellia is one of the four main oil-bearing trees (palm, coconut, olive, and tea) in the world (Anon, 2007).
Camellia oleifera C. Abel, which originated in southern China, is notable as an important source of edible oil obtained from its seeds. It is commonly known as the oil-seed camellia or tea oil camellia, though to a lesser extent other species of camellia are used in oil production too. One of the other tea trees, Camellia japonica, is distributed in Korea, China, Taiwan, and Japan.
Previous studies have revealed that Camellia oleifera has significant biological activities and includes various kinds of chemical constituents such as terpenoids and flavonoids in its leaves, roots, seeds and fruit shells (Xiong et al., 2018). Also Luan et al. (2020) has reported on the recent advances in Camellia oleifera C. Abel through a review of nutritional constituents, bio-functional properties, and potential industrial applications.
But there is a very little study on the camellia nut shell extractives even in China.
Several researches have previously reported a couple of papers on the isolation and structure determination of gallotannins from domestic tree species (Kwon and Bae, 2009; Lee and Bae, 2015; Lee et al., 2016a; Lee et al., 2016b).
Recently some domestic researchers also have evaluated on the biological activities of pine and oak tree species (Masendra et al., 2019; Mun et al., 2020; Mun et al., 2021; Jung et al., 2017; Yang et al., 2019; Min et al., 2019; Manurung et al., 2019).
However, there was no chemical constituent study on the species, including domestic camellia tree (Camellia japonica) extractives, although several papers have reported on the antioxidative and anti-inflammatory activities.
Furthermore, there is no research at all on the chemical constituent of camellia nut shell which is one of agricultural waste.
Therefore, this study was to investigate the chemical constituents, especially hydrolysable tannins, of nut shell extractives of Chinese Camellia oleifera, to promote international cooperative research between countries on wood extractives study and to provide basic information for the utilization of domestic camellia extractives in the future through this study.
2. MATERIALS and METHODS
The nut shell of camellia tree (Camellia oleifera) was obtained from Fenyi county Camellia Oil Company in Xinyu, Jiangxi Province, China. The materials were air-dried at room temperature for 2 weeks and ground (150-200 mesh).
1H and 13C NMR spectra were recorded on a Bruker Avance DPX 400 MHz spectrometer using tetramethylsilane (TMS) as an internal standard, and chemical shifts are given in δ (ppm). FAB-MS was conducted using an Autospec M363 spectrometer (Micromass, Manchester, UK). Column chromatography was done on a lipophilic Sephadex LH-20 (25-100 um, sigma) and silica gel (230-400 mesh, sigma) columns. Eluents were collected using a fraction collector (Gilson, FC 204). Thin layer chromatography (TLC) was performed on DC-Plastikfolien Cellulose F (Merck) plates and developed with TBAW (t-BuOH-HOAc-H2O, 3:1:1, v/v/v) and 6% aqueous HOAc. Visualization was done under UV light (254 and 365 nm) followed by heating after spray of vanillin-HCl (vanillin:HCl:EtOH, 4.8:12:480 (w/v/v)) solution.
The air-dried, ground nut shell of camellia tree (20 kg) was immersed in 95% EtOH at room temperature for 3-5 days. After filtration, above extraction was repeated 3 times. The filtrates were combined together and concentrated on a rotary evaporator under the reduced pressure at 40 ℃. The aqueous residue (260 g) was successively fractionated on a separatory funnel and freeze dried to give n-hexane (29 g), CH2Cl2 (24 g), EtOAc (63 g), n-BuOH (21 g) and H2O (136 g) soluble fractions.
A portion of EtOAc fraction (28 g) was chromatographed on a silica gel column using EtOAc : n-hexane mixtures (1:3→1:1 →2:1) as eluting solvents to afford 8 fractions: EF-1 (4.6 g), EF-2 (1.4 g), EF-3 (0.8 g), EF-4 (2.7 g), EF-5 (3.1 g), EF-6 (2.3 g), EF-7 (2.8 g) and EF-8 (2.7 g). EF-2 was retreated on a Sephadex LH-20 column with MeOH-H2O mixtures (1:1→1:3) to isolate compound 1 (0.72 g). EF-4 was also rewashed on a Sephadex LH-20 using MeOH- H2O (1:4) followed by EtOH: n-hexane (2:1) and then MeOH-H2O (3:1) to give 78 mg of compound 2. EF-6 was also rechromatographed on a Sephadex LH-20 using MeOH-H2O (1:3→1:1→2:1) and finally with 100% methanol to get compound 4 (93 mg). EF-8 was washed again on a Sephadex LH-20 using MeOH-H2O mixtures (1:3→1:1→2:1→100% MeOH) and finally with MeOH-H2O (3:1) to afford compound 3 (134 mg).
Brown amorphous powder, Rf: 0.54 (TBAW) and 0.41 (6% HOAc), FAB-MS: calculated for C7H6O5, 170, found m/z 171 [M+H]+, 1H-NMR (MeOH-d4): 7.1 (2H, s, H-2, 6). 13C-NMR ((MeOH-d4): 109.9 (C-2, 6), 122.4 (C-1), 138.6 (C-4), 145.8 (C-3, 5), 170.8 (C-7).
Brown amorphous powder, Rf: 0.38 (TBAW) and 0.33 (6% HOAc), FAB-MS: calculated for C20H20O14, 484, found m/z 485 [M+H]+. 1H-NMR (MeOH-d4): 3.54-3.74 (2H, H-4, 5), 4.36 (1H, dd, J=5, 12 hz, glu H-6), 4.60 (1H, dd, J=2, 12 hz, glu H-6), 5.15 (2H, glu H-2, 3), 5.71 (1H, d, J=8Hz glu H-1), 7.12, 7.16 (each 2H, S, galloyl H-2, 6). 13C-NMR ((MeOH-d4): 95.4 (glu C-1), 73.2 (glu C-2), 77.0 (glu C-3), 70.6 (glu C-4), 75.5 (glu C-5), 64.3 (glu C-6), 120.1, 120.9 (galloyl C-1), 110.0, 110.3 (each 2C, galloyl C-2, 6), 145.8 (4C, galloyl C-3, 5), 139.1, 139.5 (galloyl C-4), 166.3, 166.5 (galloyl C=O).
White brown amorphous powder, Rf: 0.25 (TBAW) and 0.10 (6% HOAc), FAB-MS: calculated for C27H24O18, 636, found m/z 637 [M+H]+. 1H-NMR (MeOH-d4): 3.67-3.85 (2H, dd, glu H-4, 5), 4.48 (1H, dd, J=5, 12 hz, glu H-6), 4.58 (1H, br d, J=12 hz, glu H-6), 5.24 (2H, t, J=9 Hz, glu H-2, 3), 5.93 (1H, d, J=8 Hz, glu H-1), 7.04, 7.08, 7.15 (each 2H, s, galloyl H-2, 6). 13C-NMR ((MeOH-d4): 94.1 (glu C-1), 74.3 (glu C-2), 75.9 (glu C-3), 71.4 (glu C-4), 76.6 (glu C-5), 64.2 (glu C-6), 120.1, 121.1, 121.3 (galloyl C-1), 110.3, 110.5, 110.6 (each 2C, galloyl C-2, 6), 146.4 (6C, galloyl C-3, 5), 138.8, 139.0, 139.5 (galloyl C-4), 166.5, 167.6, 168.3 (galloyl C=O).
White brown amorphous powder, Rf: 0.16 (TBAW), 0.19 (6% HOAc), FAB-MS: calculated for C34H28O22, 788, found m/z 789 [M+H]+. 1H-NMR (MeOH-d4): 4.54 (1H, dd, J=5, 12 hz, glu H-6), 4.63 (1H, dd, J= 12 hz, glu H-6), 6.12 (1H, d, J=8Hz, glu H-1), 5.60 (1H, dd, J=8Hz, glu H-3), 5.46 (1H, dd, J=8Hz, glu H-2), 3.98-4.03 (2H, m, J=8Hz, glu H-4, 5), 6.95, 7.03, 7.05, 7,14 (each 2H, s, galloyl H-2, 6). 13C-NMR (MeOH-d4): 93.8 (glu C-1), 72.3 (glu C-2), 76.4 (glu C-3), 79.6 (glu C-4), 76.5 (glu C-5), 63.9 (glu C-6), 119.8, 120.4, 120.9, 121.2 (galloyl C-1), 110.2, 110.4, 110.5, 110.6 (each 2C, galloyl C-2, 6), 146.3-146.6 (8C, galloyl C-3, 5), 139.9-140.6 (4C, galloyl C-4), 166.3, 167.1, 167.7, 168.1 (4C, galloyl C=O).
3. RESULTS and DISCUSSION
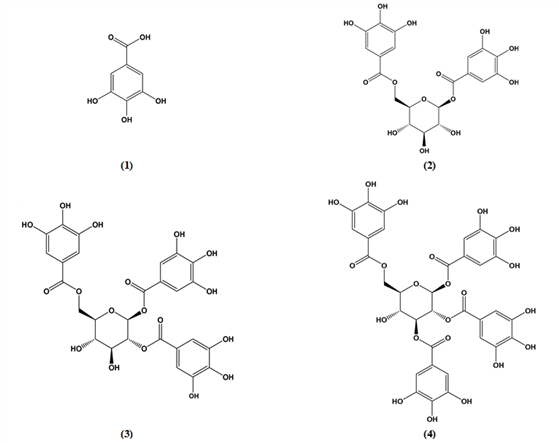
Compound 1 was obtained as a brownish amorphous powder from the EtOAc soluble fraction. The molecular formula of C7H6O5 was supported by an ion peak at m/z 171 [M+H]+ in the FAB-MS spectrum. 1H-NMR gave one signal at δ 7.1, indicating a pair of symmetrical galloyl protons, H-2 and H-6. 13C-NMR showed 7 carbon signals as already mentioned in 2.3.1. Therefore, compound 1 was identified as gallic acid (3,4,5-trihydroxy benzoic acid) (Luo et al., 2009; Kashiwada et al. 1988; Saijo et al., 1990).
Compound 2 was brown amorphous powder and contains two galloyl groups as indicated by the 1H-NMR and 13C-NMR spectra. In the 1H-NMR spectrum, compound 2 appeared the doublet anomeric proton signal shifted considerably downfield at δ 5.71 (J=8 Hz), indicating that the galloyl is connected to this position through an ester linkage and with β mode. Another galloyl was concluded to be present at the glucose C-6 position by the significantly deshielded signals, attributable to the glucose two H-6 (δ 4.36 and δ 4.60, dd) signals and clear different from other sugar signals.
The 13C-NMR spectrum showed the typical glucose signals, including C-1 at 95.4 ppm and C-6 at 64.3 ppm. The galloyl carbonyls gave 2 signals at 166.3 and 166.5 ppm. Two pairs of symmetric carbons indicated at 110 and 110.3 ppm (4C, C-2 and C-6) and at 145.8 ppm (4C, C-3 and C-5), respectively.
Above NMR data were similar to the previous reported literatures (Tanaka et al., 1982; Nonaka and Nishioka, 1983; Li et al., 2015) and the compound was determined as 1,2-di-O-galloyl-β-D-glucopyranoside.
Compound 3 was white brown amorphous powder and contains three galloyl groups as appeared by the 1H- and 13C-NMR data.
The 1H-NMR spectrum showed three symmetric galloyl group signals at δ 7.04, δ 7.08 and δ 7.15 (each 2H) and also six signals of the glucose moiety at δ 6.93 (H-1), δ 5.24 (H-2, H-3), δ 4.48-4.58 (H-6), and δ 3.67-3.85 (H-4, 5). The location of the three galloyl in the glucose moiety were decided to be the C-1, C-2 and C-6 by the analysis of the 13C-NMR data that was notable for the deshielding of the corresponding H-1 (δ 5.93, d, J=8.4 Hz), H-2 (δ 5.24, t, J=9.1 Hz) and H-6 (δ 4.48-4.58, dd, J=4, 12 Hz), being assignable to the protons germinal to galloyl groups.
The 13C-NMR spectrum indicated six signals for the glucose moiety at 64.2-94.1 ppm and three carbonyls of the galloyl groups at 166.5, 167.6 and 168.3 ppm. One three symmetric carbons gave three signals at 110.3, 110.5 and 110.6 ppm for symmetric C-2 and C-6, respectively and another three symmetric carbons gave the signals at 146.3-146.4 ppm for symmetric C-3 and C-5.
These NMR spectra were identical to the report by Nonaka et. al. (1981) and compound 3 was elucidated to 1,2,6-tri-O-galloyl-β-D-glucopyranoside.
Compound 4 was isolated as a yellowish amorphous powder from the EtOAc soluble fraction of camellia nut shell extractive. The molecular formula of C34H28O22 was supported by an ion peak at m/z 788 [M+H]+ in the FAB-MS spectrum.
In 1H-NMR spectrum, anomeric proton H-1 containing one galloyl group gave a doublet signal at δ 6.12, indicating β-structure with J=8.2 Hz. The other galloyl containing H-2 and H-3 appeared at δ 5.46 and δ 5.60, respectively. The fourth galloyl containing H-6 gave two double doublet signals at δ 4.54 and δ 4.63.
These 1H-NMR spectra were identical to the literature (Duan et al., 2004; Owen et al., 2003; Saijo et al., 1989).
In 13C-NMR spectrum, six carbons of the glucose unit resonated at 63.9-93.8 ppm. Anomeric C-1 gave a signal at 93.8 ppm and C-6 appeared at 63.9 ppm. Four carbonyls of the galloyl groups showed four signals at 166.3-168.1 ppm. One four pairs of symmetric carbons (C-2 and C-6) gave the signals at 110.2-110.6 ppm and another four pairs of symmetric carbons (C-3 and C-5) showed four signals at 145.3-146.6 ppm. These 13C-NMR data were the same as the previous reported data (Duan et al., 2004; Owen et al., 2003; Cui et al., 2002; Lee, 2016).
Therefore, this compound was identified as 1,2,3,6- tetra-O-galloyl-β-D-glucopyranoside.
In general, gallotannins are reported to have excellent biological activities such as antioxidation and antiinflammation, and it will be necessary to evaluate the biochemical properties on the isolated compounds for the chemical application in the future.
4. CONCLUSIONS
Three gallotannins, 1,2-di-O-galloyl-β-D-glucopyranoside (2), 1,2,6-tri-O-galloyl-β-D-glucopyranoside (3) and 1,2,3,6-tetra-O-galloyl-β-D-glucopyranoside (4), including gallic acid (1), were isolated from the EtOAC fraction of the nut shell extractives of Camellia oleifera. The isolation was done by column chromatography using silica gel and Sephadex LH-20 as column packing gels. The column washing solvents were MeOH, aqueous MeOH and EtOH-n-hexane. Structure determination was done by NMR and FAB-MS spectroscopy. Although nothing new, these gallotannins were first reported from the nut shell extractives of Camellia oleifera C. Abel. Then it will be necessary to evaluate the biochemical properties on the isolated compounds for future use.