1. INTRODUCTION
Recently, as the respiratory syndrome caused by SARS-CoV-2 infection, known as COVID-19, has become more serious, the World Health Organization (WHO) declared it as a pandemic, the highest level of risk assessment. In addition, the WHO announced that the number of infections caused by COVID-19 worldwide exceeded 36 million and that the mortality rate also reached 3.5 %. While the development of vaccines against COVID-19 is undergoing, it is difficult to distribute them due to the prolonged clinical trials and there are no preventive measures proposed by center for disease control (CDC) other than practicing personal hygiene, such as washing hands and wearing masks. However, skin diseases such as dermatitis, eczema, and hives, etc. could be caused by wearing a mask for an extended time, and in particular, there is a concern about a skin disorder in humid conditions caused by Candida albicans, a pathogenic yeast (Mary et al., 1968). Also, due to the scarcity of masks, a secondary infection by other pathogenic microorganisms from re-using the masks is a threat to the public health (Ravine et al., 2020).
C. albicans is an opportunistic fungus that resides in human body and it multiplies and causes inflammation when the immunity weakens. Vaginal Candidiasis is a typical disease caused by C. albicans infection and symptoms such as watery vaginal discharge, painful urination, dyspareunia, and burning sensation may occur (Park et al., 2018). Once C. albicans proliferate in the oral mucosa, the oral candidiasis may occur, causing white pseudomembrane to from in the oral cavity and macerated mucosa leads to bleeding (Mayer et al., 2013). Also, candidemia is a disease with high prevalence and mortality, which is known to be caused mostly by C. albicans (Trick et al., 2002).
Meanwhile, it has been reported that COVID-19 infection promotes cell aging by increasing oxidative stress along with diseases such as aging, diabetes, cancer and hypertension (Marie et al., 2020). Oxidative stress induced by viral infection was first published in the Sendai virus infection study in 1979 (Peterhans et al., 1979). NADPH-linked dehydrogenase is activated through the binding of the virus with the cells to generate reactive oxygen species (ROS) such as superoxide anion and hydrogen peroxide, which induces oxidative stress (Zhang et al., 2019). These free radical species generated through this process can cause skin aging and also act as a risk factor for cancers or lifestyle diseases (Sies, 2003).
A number of studies have been conducted on substances with antioxidant and antimicrobial activities. In addition to these studies, synthetic drugs with antioxidant and antimicrobial activities have been continuously developed (Sen et al., 2010). Representative antioxidants include ethoxyquin, propyl gallate (PG), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and other phenolic compounds, which are used as cosmetic preservatives (Chen et al.,1992). Representative antimicrobial agents are ketoconazole, itraconazole, and terbinafine, etc. (Mattew et al., 1993). Ketoconazole is used to treat diseases caused by fungi such as ringworm, candidiasis, and seborrheic dermatitis (Min et al., 1992). Itraconazole is an antimicrobial used to treat onychomycosis, candidal vaginitis, dermatophytosis, mycotic keratitis, and oral candidiasis and is effective against fungi such as C. albicans and Aspergillus fumigatus (Denning et al., 1997). Terbinafine is mainly used to treat the mycotic infection on nails, but it has been reported that terbinafine also shows antimicrobial activity against Trichophyton rubrum, which causes acne (Mukherjee et al., 2003).
Despite these effects, however, long-term use of synthetic compounds may cause various side effects, including kidney toxicity, headache, and dermal hypersensitivity (Del et al., 2005). Thus, there has been a demand for the development of antioxidants and antimicrobial agents that are not harmful to the human body with minimized side effects. Accordingly, interest in substances derived from natural resources is increasing (Koehn et al., 2005). Among natural substances, plant essential oils have been reported to have diverse composition with physiological activities. A typical natural product, plant essential oils are volatile organic compounds extracted from flowers, leaves, stems, and roots and are secondary metabolites, which are essential for plants to survive. It is known that such plant essential oils are composed of terpene compounds, mainly monoterpenes, and have antioxidant and antimicrobial effects (Luis et al., 2019; Sharma et al., 2017).
Recently, interest in cypress tree is increasing, which contains vast amount of phytoncide, a substance exhibiting antimicrobial activity in plant essential oils. The essential oil of cypress shows antioxidative as well as antimicrobial activities against Listeria monocytogenes, T. rubrum, etc. and was evaluated to be safe in cytotoxicity tests. Beside the cypress tree, essential oils of eucalyptus, Japanese red pine, and Korean white pine have been reported to have anti-oxidative, anti-inflammatory, and anti-allergic effects (Seo et al., 2015). Japanese larch essential oil is also known to have anti-fungal activity against Pidermophyton floccosum, Trichophyton mentagrophytes, and T. rubrum, which are dermatophytes (Kim et al., 2013). The commercial value of these plant essential oils with high physiological activity has continuously increased. However there is a limit, in which they do not have antimicrobial activity against all strains, and for South Korea, most of the raw materials of the essential oils are being imported.
Therefore, in this study, we established basic data for the development of natural antioxidants and antimicrobial agents by evaluating antioxidant and antimicrobial activity of 7 domestic plant essential oils that have not been studied in South Korea. We also aimed to identify active components based on domestic plant essential oils, which have both antioxidant and antimicrobial activities.
2. MATERIALS and METHODS
The domestic wild species used in this study are Cinnamomum loureiroi Nees, Juniperus chinensis, Zanthoxylum schinifolium S. et Z., Zanthoxylum piperitum, Artemisia capillaris Thunb., Citrus unshiu, Citrus pseudogulgul hort. and the collection areas are shown in Table 1. Leaves, fruits, and fruit shells were stored frozen at –39°C in green condition for hydrodistillation. We evaluated the antioxidant activity on 7 plant essential oils and the antimicrobial activity was evaluated by selecting essential oils showing the antioxidant activity. C. albicans (ATCC 10231), which causes skin diseases, was obtained from the Korea Culutre Center of Microorganism, and cultured in a sabouraud dextrose agar (SDA, BD ProbeTec™ ET, Becton–Dickinson Microbiology System, Sparks, MD, USA) medium at 26~28°C for 4 days.
Each plant essential oil was extracted by hyro-distillation. 1 kg of the test plant sample was added to a 10 L round-bottom flask, and distilled water was added to the extent that the sample was immersed. Then, the plant essential oils were collected using a 102±1°C heating mantle (MS-DM608 heating mantle, MTOPS®, Yangju, Korea) and a dean stark trap. Moisture remaining in the essential oils was removed with anhydrous sodium sulfate (Samchun, 98.5%, Korea) and the obtained essential oils were stored in a refrigerator at 4°C.
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging ratio was measured by modifying the methods of Blois (1958) and Sharma (2009). After dissolving DPPH in 95% ethanol (Samchun, Korea) to a concentration of 0.1 M, it was mixed 1:1 with the essential oil. The final concentration of the solution was adjusted to be 3, 2, 1, 0.5, 0.25, and 0.125 mg/mL and reacted in the darkroom for 30 minutes. The absorbance was measured using a UV-Vis spectrophotometer (Shimadzu UV-1601 PC spectrophotometer, kyoto, Japan) at a wavelength of 517 nm. DPPH radical scavenging ratio was calculated using the following equation (1). Also, a calibration curve was plotted in the range of 0-100 μM for the positive controls, trolox, ascorbic acid, and tocopherol. Based on this, the equivalent amount of the plant essential oil to the standard substance was estimated in the unit of SC50 value of standard substance (μmol) versus SC50 value of plant essential oil (per 100g).
As: Absorbance of the sample
Ac: Absorbance of the control
2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity was measured by modifying the methods of Re (1999) and Konan (2016). 8 mL of 7 mM ABTS and 2 mL of 12.25 mM aqueous potassium persulfate solution (Samchun, Korea) were mixed and reacted in a darkroom at 37°C for 16 hours. Then, the mixture was diluted with ethanol so that the absorbance at a wavelength of 734 nm was 0.70 (±0.02). The diluted ABTS•+ solution and essential oil were mixed at 24:1 ratio, which was adjusted to the final concentration of 2, 1, 0.5, 0.25, and 0.125 mg/mL for the essential oil. Finally, after the mixture was reacted in the darkroom at 37°C for 5 minutes, the absorbance was measured using a UV-Vis spectrophotometer at a wavelength of 734nm. ABTS radical scavenging ratio was calculated using the following equation (2). Also, a calibration curve was plotted in the range of 0-100 μM for the positive controls trolox, ascorbic acid, and tocopherol. Based on this, the equivalent amount of the plant essential oil to the standard substance was estimated in the unit of SC50 value of standard substance (μmol) versus SC50 value of plant essential oil (per 100g).
As: Absorbance of the sample
Ac: Absorbance of the control
On a thin layer chromatography (TLC) plate (TLC silica gel 60 F254, merck, 20 × 20 cm, 0.2 mm thickness) eluted with n-Hexane:Ethyl acetate (8:1) solution, DPPH or ABTS radical solution was sprayed to visualize the group of active substances. Based on the stained TLC plate, the ratio of the distance moved by the active substance group to the distance moved by the eluent (Rf value) was calculated. prep. TLC (TLC silica gel 60 F254, merck, 20 × 20 cm, 0.2 mm thickness) was conducted using the same eluent and the corresponding substances were extracted using ethyl acetate from silica gel based on the Rf value. For the quantitative and qualitative analysis of the extracted substances, GC/MS component analysis was performed.
To evaluate the antimicrobial activity of the plant essential oils, the disk diffusion assay was conducted according to the protocol suggested by the Clinical and Laboratory Standards Institute (CLSI, USA). To prepare a solution at a concentration of 1×106 CFU/mL (0.5 McFarland turbidity standard), C. albicans was diluted with distilled water and adjusted to absorbance of 0.12~0.15 at a wavelength of 530 nm by using a UV-vis spectrometer (Shimadzu, Japan). After inoculating the diluted C. albicans inoculum into a Mueller-Hinton agar supplemented with 2% glucose and methylene blue (MH-GMB, 0.5 μg/ml) medium, a paper disk with a diameter of 8 mm in which 20 μL of 100 and 50 mg/mL essential oil was absorbed, was placed and cultured at 26~28°C for 4 days. The diameter of the clear zone formed around the disk was measured, and the central part of the disk and the boundary of the clear zone were collected to observe the change in the cell morphology of C. ablicans using the scanning electron microscope (SEM).
To investigate the minimum inhibitory concentration (MIC) of C. albicans, microdilution was performed according to the protocol suggested by CLSI. The 1×106 CFU/mL C. albicans solution (0.5 McFarland turbidity standard) was diluted 1000 times to adjust the concentration to 1×103 CFU/mL. 100 μL of YB broth medium was dispensed to a 96-well plate and 100 μL of the essential oil was dispensed for each concentration. Then, 100 μL of the diluted C. albicans inoculum was added, followed by incubation at 26~28°C for 4 days to measure the MIC.
SEM analysis was conducted by modifying the method suggested by Insuan & Chahomchuen (2020). After inoculating C. albicans to SDA, an 8 mm paper disk absorbed with 20 μL of 100 mg/mL essential oil was placed and incubated at 26~28°C for 4 days. The central part and the boundary part of the clear zone around the disk were collected and attached to aluminum stub using carbon tape, followed by the treatment with 2% osmium tetroxide vapor for 24 hours to fix C. albicans. After coating with gold for 2 minutes using Gold sputter, the surface area change of C. albicans was observed at a magnification of 10,000~20,000 using SEM (Supra 55vp, Carl Zeiss, Germany).
The essential oils used in this study are a mixture of various organic substances. Among these substances, TLC bioassay was conducted to detect the major components having antimicrobial activity. Plant essential oils were eluted on a TLC plate using n-Hexane:Ethyl acetate (8:1) as an eluent and observed at UV 254 nm to divide the active fraction. The eluted TLC plate was cut along the elution direction and placed on a solid medium 5 minutes after the C. albicans inoculation. Then, it was cultured at 26~28°C for 4 days to identify the clear zone and the fraction groups with the same Rf value as the active fraction were extracted. Finally, GC/MS component analysis was conducted.
For the GC/MS component analysis in this study, all samples were analyzed according to the following conditions using tridecane as an internal standard. DB-5 column (25 m × 0.32 mm × 0.52 μm) was used for the GC (model-Agilent 7890, USA) analysis. Helium was used as the carrier gas. The injector temperature was 260°C and the detector temperature was 280°C. The oven temperature was maintained at an initial temperature of 50°C for 5 minutes and then increased by 5°C/min to reach the final temperature of 250°C and maintained for 10 minutes. For mass spectrometry, Agilent 5937 was used and it was analyzed in EI mode. The structure of a compound was identified by comparing the mass data of the obtained sample peak with the standard library data (Willy 7th ed).
3. RESULTS and DISCUSSION
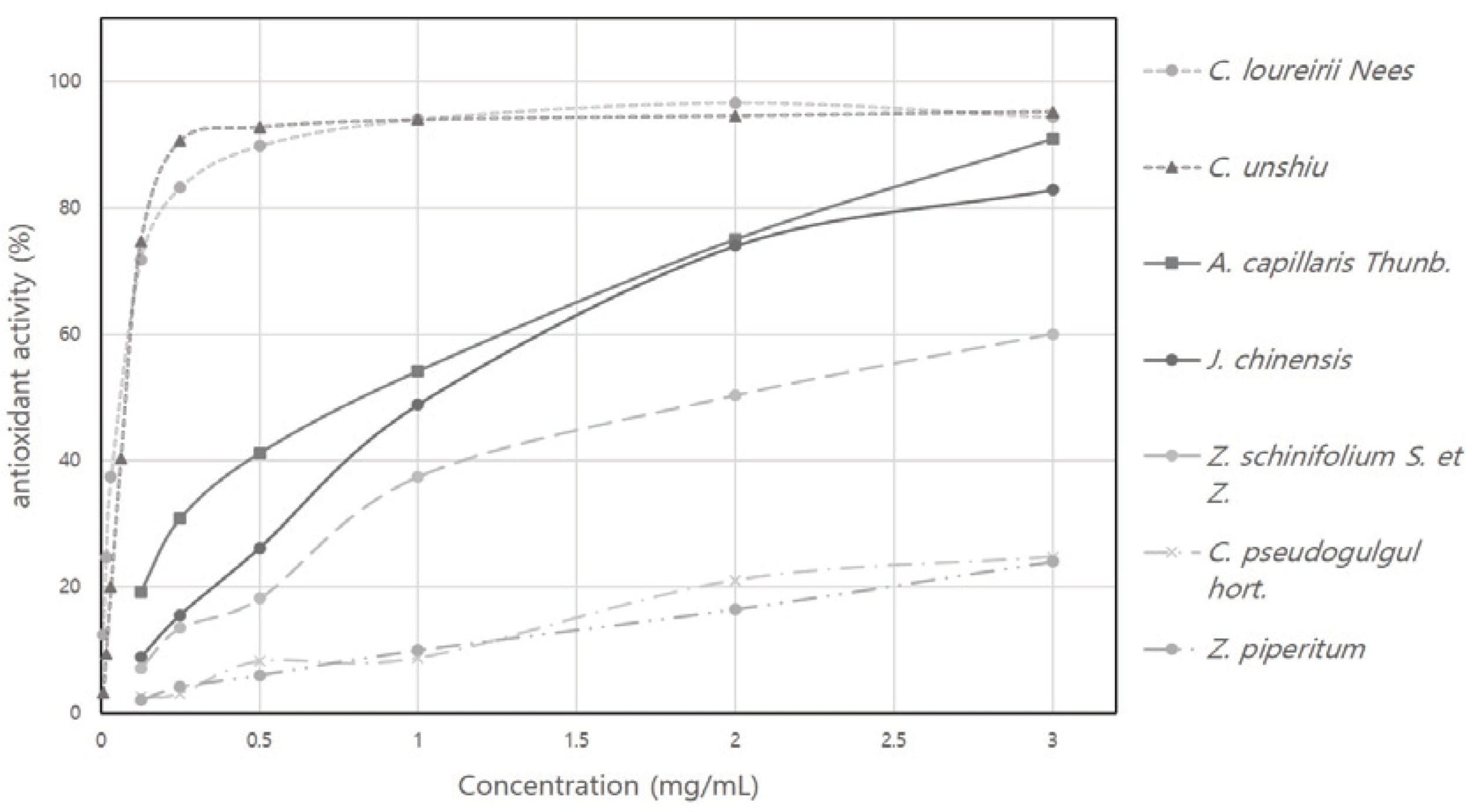
The DPPH radical scavenging activity was measured for C. loureirii Nees, J. chinensis, Z. schinifolium S. et Z., Z. piperitum, A. capillaris Thunb., C. unshiu, and C. pseudogulgul hort. to investigate the antioxidant activity of the plant essential oils and the results are shown in Fig. 1. The DPPH radical scavenging activity is an antioxidant index for phenolic substances such as flavonoids and phenolic acids and it is known that the higher the reducing power, the higher the radical scavenging activity (Kang et al., 1995). Among 7 plant essential oils, the DPPH radical scavenging activities of the essential oils of C. unshiu and C. loureirii at a concentration of 3 mg/mL were 95% and 94%, respectively, showing superior antioxidant activity compared to 24% for C. pseudogulgul essential oil, 91% for A. capillaris, 23% for Z. piperitum, 83% for J. chinensis, and 60% for Z. schinifolium.
Using trolox (water-soluble, vitamin E analog), ascorbic acid (vitamin C), and tocopherol (vitamin E), which are currently used as antioxidants, as positive controls, SC50 value, the concentration when the DPPH radical scavenging activity reaches 50%, and the equivalent compared to the standard were calculated. Table. 2 shows the SC50 values of the positive controls and the sample plant essential oils. The SC50 values of each plant essential oils were as follows: 0.82 mg/mL for A. capillaris, 1.25 mg/mL for J. chinensis, 1.98 mg/mL for Z. schinifolium, 7.41 mg/mL for C. pseudogulgul, 8.42 mg/mL for Z. piperitum, 0.10 mg/mL for C. unshiu, and 0.09 mg/mL for C. loureirii. That is, the SC50 values of the C. unshiu and C. loureirii essential oils were lower than those of other essential oils. Also, when comparing the equivalent compared to the standard substance, the C. unshiu essential oil had an equivalent of trolox 4.80, ascorbic acid 3.28, and tocopherol 0.3, while the C. loureirii essential oil had an equivalent of trolox 5.35, ascorbic acid 3.65, and tocopherol 0.42. In other words, the equivalent of the C. unshiu and C. loureirii essential oils were higher than that of other 5 plant essential oils. Based on these results, it is thought that the essential oils of C. unshiu and C. loureirii have high antioxidant activity. This is a similar result to the previous studies (Kim, 2013; Yang et al., 2012) in which the maximum DPPH radical scavenging activity of C. unshiu was 95% and the SC50 value of the C. loureirii essential oil was 0.073 mg/mL.
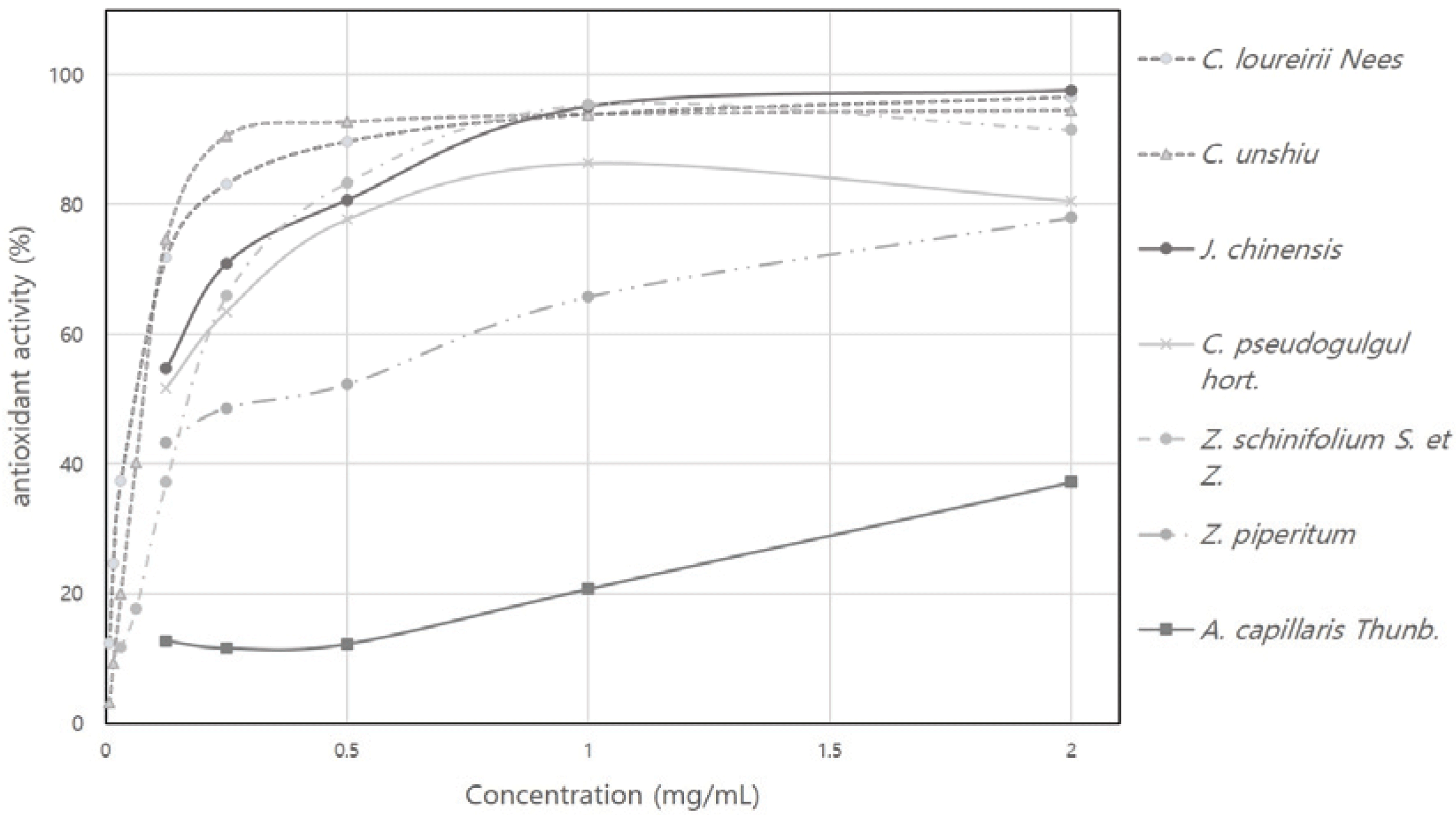
In addition to the DPPH radical scavenging activity, ABTS radical scavenging activity was measured to investigate the antioxidant activity of 7 plant essential oils and the results are shown in Fig. 2. Among 7 plant essential oils, the ABTS radical scavenging activities of the essential oils of C. unshiu, C. loureirii, and J. chinensis at a concentration of 3 mg/mL were 95%, 96%, and 97%, respectively, showing superior antioxidant activity compared to 80% for C. pseudogulgul, 37% for A. capillaris, 78% for Z. piperitum, and 91% for Z. schinifolium.
For each plant essential oil, the SC50 values and the equivalent to the positive controls trolox, ascorbic acid, and tocopherol were calculated, and the results are shown in Table. 3. The SC50 values of the plant essential oils were as follows: 0.08 mg/mL for J. chinensis, 0.25 mg/mL for Z. schinifolium, 0.10 mg/mL for C. pseudogulgul, 0.29 mg/mL for Z. piperitum, 0.09 mg/mL for C. unshiu, 0.06 mg/mL for C. loureirii, and 4.05 mg/mL for A. capillaris. That is, the SC50 values of C. unshiu and C. loureirii essential oils were relatively lower than those of other essential oils. Also, the equivalent of J. chinensis essential oil to the standard substance was trolox 4.33, ascorbic acid 2.26, and tocopherol 2.97, while that of C. unshiu essential oils was trolox 2.69, ascorbic acid 2.05, and tocopherol 3.92, and that of C. loureirii essential oil was trolox 3.94, ascorbic acid 3.01, and tocopherol 5.75. In other words, the equivalent of these three essential oils was relatively higher than that of the other essential oils. Based on these results, it is suggested that the essential oils of J. chinensis, C. unshiu, and C. loureirii have a high antioxidant activity.
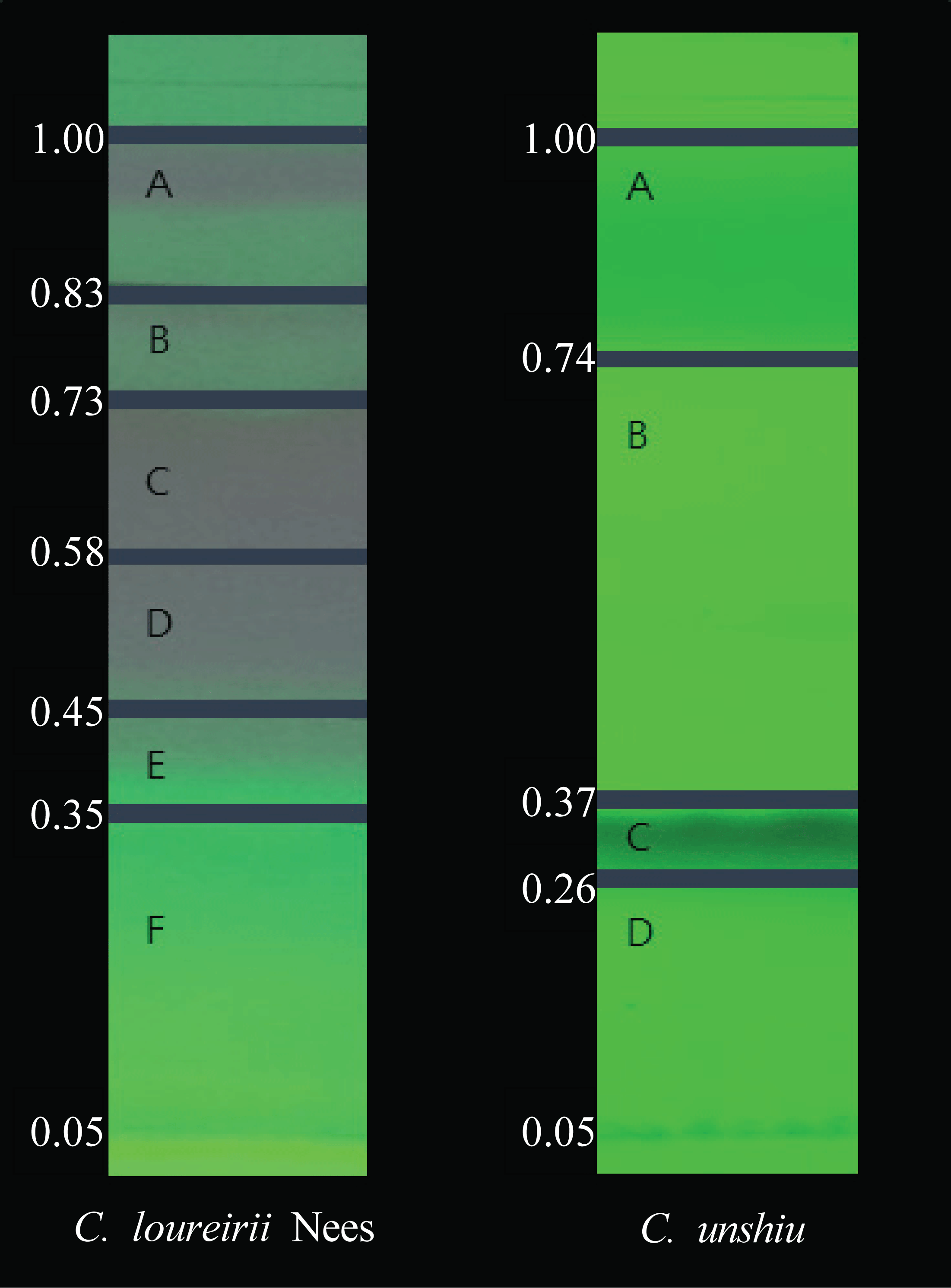
Table 4 shows the component analysis results of C. loureirii and C. unshiu essential oils. The main components of C. loureirii essential oil were linalool (21.27%) and α-citral (8.62%), while those of C. unshiu essential oil were limonene (69.87%) and γ -terpinene (6.83%). These results are similar to the previous studies in which linalool was detected in C. loureirii essential oil, and limonene and γ-terpinene in C. unshiu essential oil (Xiao et al., 2013; Seshadri et al., 2020). The essential oils of C. loureirii and C. unshiu were eluted on a TLC plate using n-Hexane: Ethyl acetate (8:1) as an eluent. After dividing the fraction as shown in Fig. 1, the Rf values were assigned.
Through the evaluation of DPPH radical scavenging activity and ABTS radical scavenging activity, it was found that the essential oils of C. unshiu and C. loureirii showed the antioxidant activity with a low SC50 value. C. unshiu essential oil showed the antioxidant activity at C. unshiu B and C. loureirii essential oil showed the antioxidant activity at C. loureirii C, D, and E. Table 5 shows the results of GC/MS component analysis on the fractions with antioxidant activity. Components such as β-myrcene, linalool, and elemol were detected in C. unshiu B. Cinnamyl acetate, eucalyptol, linalool, and citral were detected as the main components of C. loureirii. In C. loureirii C, eucalyptol, α-Citral, and geranyl acetate, while linalool and α-citral in C. loureirii D, and linalool, β-citral, α-citral, and cinnamyl acetate in C. loureirii E. Fig. 3.
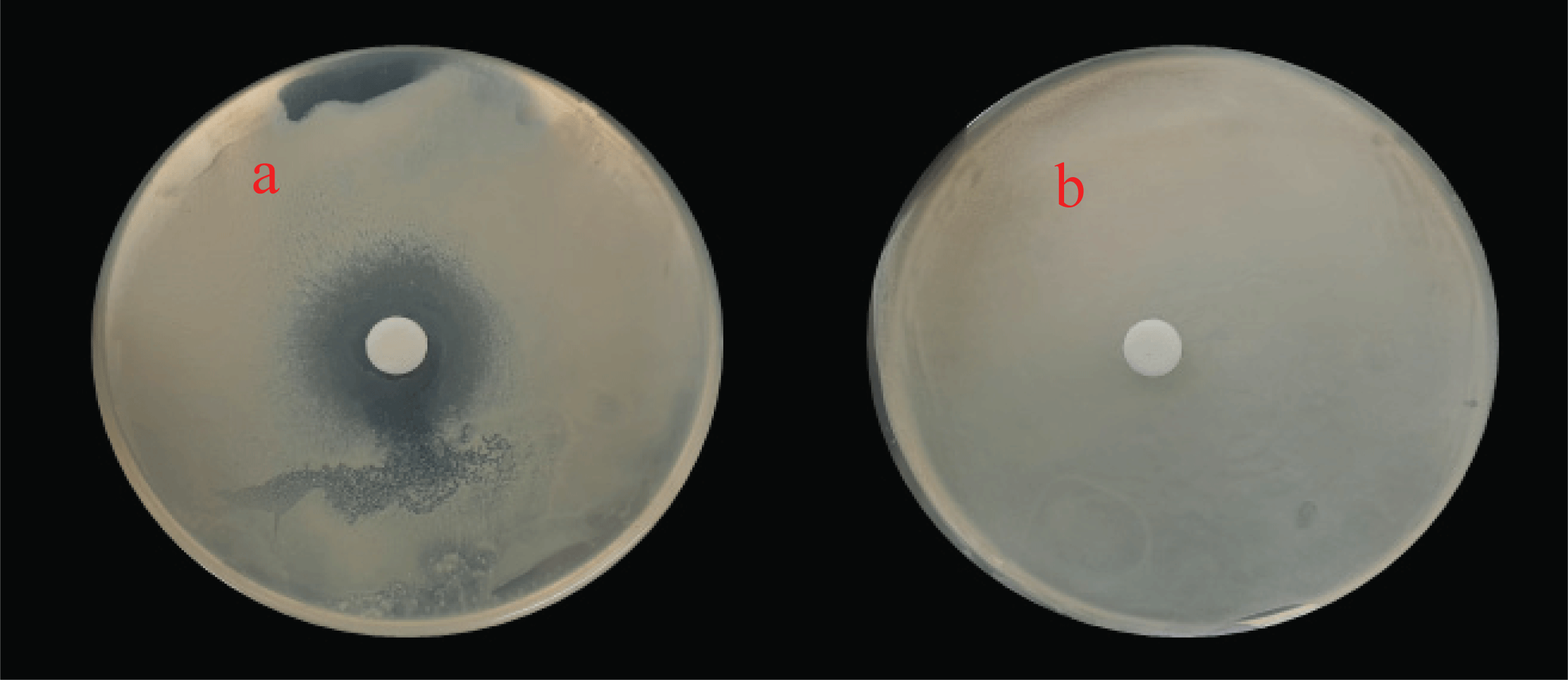
It is known that a plant essential oil has antimicrobial activity when the diameter of the clear zone around the 8 mm paper disk increases, which is absorbed with a plant essential oil in the medium inoculated with pathogenic microorganisms. The disk diffusion method was conducted for C. unshiu and C. loureirii essential oils which exhibited excellent radical scavenging ability among 7 plant essential oils. As shown in Fig. 4(a), a clear zone was formed in C. loureirii essential oil at a concentration of 100 mg/mL. However, no clear zone was formed in C. unshiu essential oil at a concentration of 100 mg/mL and 50 mg/mL. The diameters of the clear zone of the C. loureirii essential oil were 44.9±8.9 mm at a concentration of 100 mg/mL and 33.4±5.5 mm at a concentration of 50 mg/mL. Based on these results, it was confirmed that C. unshiu essential oil cannot inhibit the growth of C. albicans, but C. loureirii essential oil has antimicrobial activity against C. albicans.
After C. albicans was exposed to the plant essential oil, it was incubated at 26~28°C for 4 days. The turbidity was visually examined, and the minimum bactericidal concentration (MBC) and MIC were measured. For C. albicans, the MIC of C. loureirii essential oil was measured low as 1.25 mg/mL, while that of C. unshiu essential oil was measured relatively high as 5 mg/mL. For the MBC, C. loureirii essential oil has a value of 1.25 mg/mL and C. unshiu essential oil has a value of 5 mg/mL. The C. loureirii essential oil shows high antimicrobial activity even compared to the essential oils of cypress, Japanese cedar, and Japanese red pine. The essential oils of cypress, Japanese cedar, and Japanese red pine all had a MIC value of 2.18 mg/mL or more against C. alibicans. Also, the MIC values of these oil were 2.18 mg/mL or more against Candida genus such as Candida krusei, Candida glabrata, Candida tropicalis, and Candida pseudotropicalis, showing lower antimicrobial activity than that of the C. loureirii essential oil (Lee et al., 2001; Lee et al., 2009). It is assumed that the essential oil of C. loureirii has a high antimicrobial activity against C. albicans, whereas the C. unshiu essential oil has a low antimicrobial activity against C. albicans.
Fig. 5 shows the SEM images to visualize the phenomenon on the strain surface in which plant essential oil inhibits the growth of C. albicans. To compare the difference in the degree of growth depending on the diffusion effect of essential oils in the medium, we compared the SEM images by dividing them into the control image of C. albicans (Fig. 5(control)), the boundary and central part of the clear zone (Fig. 5(a,b,c,d)). In case of the strain treated with the C. unshiu essential oil, the growth of the strain was not inhibited, so the strain of the part where the paper disk contacted was collected and analyzed by SEM. As a result, it was found that the surface was smooth, and growth was not inhibited as shown in the SEM images (Fig. 5(b)). On the other hand, in the case of the part collected after removing the paper disk, the strains were hardly observed, which may be attributed that the contact of the disk prevented the strain growth. In contrast, it was observed that the cell wall of C. albicans was destroyed and contracted when it was treated with the C. loureirii essential oil which has antimicrobial activity. Such result may be due to the destruction of the cell wall by the essential oil as well as the loss of internal organelles by the difference in concentration between the inside and the outside of the cell. At the boundary of the clear zone, undamaged C. albicans and damaged C. albicans were observed at the same time. In Fig. 5(c), a SEM image of the inside of the clear zone treated with the C. loureirii essential oil, it was observed that the C. albicans cells were separated from each other and contracted without connection.

The clear zone on the TLC plate was observed in the C, D, and E parts of the C. loureirii essential oil. The result of the GC/MS analysis on those parts is shown in Table 6. The main components detected in each part were as follows: cinnamyl acetate, eucalyptol, linalool, and citral for the C. loureirii essential oil, eucalyptol, α-citral, and geranyl acetate for C. loureirii C, linalool, and α-citral for C. loureirii D, and linalool, β-citral, α-citral, and cinnamyl acetate for C. loureirii E. Eucalyptol in the C. loureirii essential oil was reported to have antimicrobial activity against S. aureus and citral, which was detected in C. loureirii C, D, and E, was also reported to have antimicrobial activity (Karlović et al., 2000; Saddiq et al., 2010). In the evaluation of the anti-proliferative activity against C. Albicans, the diameter of the clear zone treated with citral was 40 mm or more, which is similar to the diameter of the clear zone treated by the C. loureirii essential oil in this study. Such result suggests that the C. loureirii essential oil has a high antimicrobial activity. In addition, it has shown excellent microbial activity against other Candida strains, and the diameters of the clear zone were 27.5 mm for Candida parapsilosis, 19.7 mm for C. krusei, and 32.6 mm for C. tropicalis (Silva et al., 2008). The essential oil of Cinnamomum verum, whose main component is cinnamyl acetate, was also reported to have antimicrobial activity against Candida albicans with a MIC values of 0.12 mg/mL (Unlu et al., 2010; de Lima Carvalho et al., 2018). Geranyl acetate was reported to have antimicrobial activity against Candida strains with a MIC value of 20 μl/mL against C. krusei, 5 μl/mL against C. parapsilosis, and 1.25 μl/mL against C. guillermondii. It also has a MIC value of 0.32 μl/mL against T. rubrum and T. mentagrophytes, showing excellent antimicrobial activity against various strains (Goncalves et al., 2012). Eucalyptol, citral, cinnamyl acetate, and geranyl acetate were found to exhibit antimicrobial activity. Linalool, which was detected at the fraction of the essential oil showing antioxidant and antimicrobial activity, was also detected in the C. unshiu essential oil, which did not show antimicrobial activity. In the previous study, as the result of the disk diffusion test using linalool on C. albicans, the diameter of the clear zone was 12 mm and the MIC value of was 1.23 mg/mL, indicating that linalool has good antimicrobial activity (Herman et al., 2016). Also, it showed high antimicrobial activity against the strains other than C. albicans; the MIC value of 0.1 mg/mL on Aggregatibacter actinomycetemcomitans, 0.8 mg/mL on Porphylomonas gingivalis, and 0.2 mg/mL on Prevotella intermedia (Hsu et al., 2013). Linalool was also detected in the C. unshiu essential oil, but it is presumed that the essential oil did not show antimicrobial activity as linalool present amounts with a low content of 0.72%.
4. CONCLUSION
In this study, we selected the essential oils of C. loureirii and C. unshiu that have excellent antioxidant activity by evaluating the antioxidant activity of 7 plant essential oils. We also investigated antimicrobial activity of two selected essential oils against C. albicans. The TLC-DPPH (ABTS) analysis was conducted with the C. loureirii and C. unshiu essential oils, and their active components were identified by GC/MS analysis. The C. unshiu essential oil was mainly composed of linalool, while the C. loureirii essential oil had eucalyptol, α-citral, β-citral, and linalool as the main components. As a result of the evaluation of antimicrobial activity for two essential oils, the C. unshiu essential oil showed no antimicrobial activity, whereas the C. loureirii essential oil exhibited high antimicrobial activity with a MIC value of 1.25 mg/mL. This is also a high value when compared to the previous study that reported the essential oils of lavender (Lavandula officinalis), rosemary (Rosmarinus officinalis), and eucalyptus (Eucalyptus globulus) had a MIC value of 5 mg/mL against C. albicans (Tampieri et al., 2005). Through the TLC-bioassay, we confirmed that the C. loureirii C, D, and E fractions have antimicrobial activity, and eucalyptol, citral, geranyl acetate, and cinnamyl acetate were detected in the active fractions. In conclusion, we suggest that the C. loureirii essential oil, which has antioxidant activity and antimicrobial activity against C. albicans would be a promising candidate for solving skin problems in the future. Particularly, active components in the C. loureirii C, D, and E fractions have potential to be developed as a functional cosmetic with both antioxidant and antimicrobial activities.