1. INTRODUCTION
Wood vinegar or pyro-ligneous acid is an aqueous liquid obtained by wood distillation under anaerobic condition (Yang et al., 2016). Wood vinegar can be produced from a wide range of lignocellulose materials, including bamboo (Mu et al., 2004). Wood vinegar is a natural inhibitor which contains numerous bioactivities. Thus, it is safe and applicable for termiticidal (Hadi et al., 2010; Oramahi and Yoshimura, 2013), antifungal (Islam et al., 2009; Okutucu et al., 2011), antimicrobial, antibacterial, (Lee, et al., 2010; Bedmutha et al., 2011) and repellent (Kiarie-Makara et al., 2010). Wood vinegar consists of polycyclic aromatic hydrocarbon compounds from the pyrolysis of the cellulose. They comprise phenol, formaldehyde, organic acid, ketones, hydrocarbon, ester, alcohols and some heterocyclic materials (Kim et al., 2008; Tascioglu et al., 2012; Haji, 2013).
As a natural product, great efforts have been taken to improve the use of wood vinegar, such as its efficacy as an anti-termite. It was found that wood vinegar from Albizia chinensis, Toona sinensis sawdust and Vitex pubescens was effectively killed Coptotermes curvignathus and Cryptotermes cynocephalus, and had a mode of action as an antifeedant to Reticulitermes speratus and Coptotermes formosanus (Oramahi and Yoshimura, 2013; Adfa et al., 2017; Arsyad et al., 2019).
The composition and application characters of wood vinegar have been reported in previous studies. In addition, Mu et al. (2004) have shown the effectiveness of bamboo vinegar on the germination and radicle growth of seed plants. Although bamboo vinegar has great potential for anti-termite, the chemical composition at different pyrolysis temperature has not yet been studied.
In this study, the termiticidal activity of bamboo vinegar against subterranean termite was evaluated. Further, the chemical components of bamboo vinegar were examined.
2. MATERIALS and METHODS
Mayan (Gigantochloa robusta Kurz.), balcoa (Bambusa balcooa Roxb.), and taiwan (Dendrocalamus latiflorus Munro) bamboo were carbonized with a laboratoryscale furnace. Rubber wood (Hevea brasiliensis) was subjected to subterranean (Coptotermes curvignathus Holmgren). The material was collected from Bogor, West Java, Indonesia.
All chemicals used in this study were analytical grade HCl (Merck), KI (Merck), Na2S2O3 (Merck), bromide chromate and phenoptalein. Glass equipment (pyrex), pyrolysis reactor with condensation equipment, oven, analytical scale, pH meter digital and burrete were used in this study.
Bamboo culms were cut to size of 5 × 5 cm and pyrolized for 4 hours. The reactor temperature was set at 300°C and 400°C. Bamboo vinegars were condensed and collected. The acidity, specific gravity, and the content of acetic acid and phenol of bamboo vinegar were then measured (Wibowo, 2012).
Rubber wood samples were cut to size of 5 cm x 5 cm × 2.5 cm in accordance to the Indonesian Standard of SNI 7207-2014. Wood specimens were then oven-dried for 24 hours in 105°C to a constant weight and reach 45% of moisture content. Afterwards, the samples were weighed (W1), measured (V1) and completely immersed in a cylindrical glass of bamboo vinegar for 24 hours. The samples were cleaned up, weighed (W2) and measured (V2) after the preservation treatment. All samples were conditioned for two weeks to a constant weight and assuring the fixation process of bamboo vinegar and wood samples. Subsequently, the specimens were weighed to obtain the oven dried weight after preservation treatment.
Untreated wood was used as the control. Five replications were made for each treatment.
The content of acetic acid in bamboo vinegar was analyzed using the AOAC method. About 10 gram of bamboo vinegar was dissolved in 100 ml distilled water. Then, phenopthalein indicator was added into 10 ml of each solution. It was titrated with 0.1 N NaOH solution. The acetic acid content was calculated using the following equation.
Remarks: V NaOH = volume of NaOH; N NaOH = normality of NaOH; and BM = molecule weight of acetic acid
The phenolic content in the bamboo vinegar was evaluated using titration with 2N NaOH used the same method as AOAC (AOAC, 1990). The phenolic content was calculated using the following equation.
Remarks: a = mL Na2S2O3 in sample solution; b = mL Na2S2O3 in a blank solution; BM = molecular weight of phenol; N = Normality of Na2S2O3; fp = dilution factor; and S = sample weight
The termiticidal activities against Coptotermes curvignathus Holmgren were examined using the Indonesian Standard of SNI 7207-2014. The wood specimen was placed in a glass jar. About 200 g of moist sand (7% moisture content) and 200 healthy and active workers of Coptotermes curvignathus Holmgren subterranean termites were added in to the jar. The glass jars then were kept in 25°C to 30°C dark room with relative humidity of 80% to 90% for four weeks. The jars were weighed every week.
After four weeks, the specimens were cleaned up and put in the oven at 60°C. Termite mortality and weight loss of wood specimens were determined using the following equation.
Remarks: T1= the number of live termites prior to the test; T2= the number of live termites after the test.
Remarks: W1= the weight (g) of oven-dried samples prior to the test; W2 = the weight (g) of oven-dried samples after the test.
It was assumed that termite mortality was linear to time. The feeding rate was calculated using the following equation.
Based on the wood weight loss percentage, wood specimens were categorised for its resistance using SNI 7207-2014.
Data analysis was performed using a 3 × 2 factorial in a completely randomized design. Bamboo vinegar and pyrolysis temperature (300°C and 400°C) were the first and second factor, respectively. Duncan’s multiple range tests were used since at p<0.05 the factor was significantly different. Microsoft Excel and Statistical Product and Service Solution version 22 were operated to analyze the data.
3. RESULTS and DISCUSSION
The properties of bamboo vinegars are shown in Table 1. The pH scale of bamboo vinegars ranged from 2.88 to 3.17. This value was used to distinguish the decomposition process of lignocellulosic raw material chemical compound (Pamori et al., 2015). This process yielded organic acid and acetate acid as the primary component of wood vinegar from cellulose and hemicellulose decomposition process (Pamori et al., 2015). The lowest pH scale of wood vinegar was expected to have superior quality (Komarayati and Wibowo, 2015). Additionally, pH scale was influenced by moisture content of the wood vinegar (Pamori et al., 2015).
As shown in Table 1, the specific gravity of bamboo vinegar was around 1.019–1.045. Specific gravity depicted molecule density in wood vinegar. Thus, it was influenced by cellulose, hemicellulose and lignin degradation and chemical content of wood vinegar (phenol, tar and polycyclic aromatic hydrocarbon (Nasruddin, 2015).
Table 1 shows total phenol and total acid in the bamboo vinegar. Total phenol ranged from 13.52% to 22.98%, while total acid content ranged from 8.33 to 12.37%. It can be seen in Table 1 that total phenol and acids in bamboo vinegar pyrolyzed at 400°C were significantly greater than bamboo vinegar pyrolyzed at 300°C, with the exclusion of total acid in D. latiflorus Munro bamboo vinegar. In agreement to this, previous studies reported that total phenol was the largest component in wood vinegar from Quercus serrata, Pinus densiflora, walnut shell and pineapple plant waste biomass (Mun and Ku, 2002; Ma, et al., 2011; Mathew et al., 2015). Phenol and acids in wood vinegars of palm kernel shells were more progressive in the condensation reaction at higher temperature (Choi et al., 2015). Acetic and benzoic acids were expeditiously escalated with the raising temperature up to 500°C, thus it confirmed that some chemical contents were affected by temperature (Heo et al., 2010). Moreover, the chemical contents of wood vinegar were also influenced by material size and the amount of cellulose, hemicellulose, lignin, volatile extractives, fixed carbon, and ash in the samples (Demiral and Ayan, 2011; Abnisa et al., 2013). In general, cellulose and lignin contents in bamboo is around 44-55% and 23-32%, respectively (Damayanti et al., 2019). Pyrolysis of cellulose yielded acetate acid, water and a small amount of phenol, while pyrolysis of lignin produced phenol and its derivatives (Salindeho et al., 2017).
Total phenol in wood vinegar was affected by lignin and pyrolysis temperatue. The high content of lignin coupled with attested temperature resulted in impeccable lignin decomposition and high phenol content (Asmawit and Hidayati, 2016). As it was stated earlier, acetate acid is one of the primary components in the wood vinegar and the highest acid content from cellulose and hemicellulose decomposition process. Thus, it confirms that the acetate acid content was influenced by raw material used in the process (Choi et al., 2012; Pamori et al., 2015).
The retention value of wood preservative shows the absorptive capacity of the preserved wood. The high value indicated the efficacy of preservative, thus wood would be easily preserved and retained from deterioration (Suheryanto, 2010). The analysis of variance shows that bamboo vinegar had significant effect to retention value, however pyrolysis temperature and the interaction between bamboo vinegar and pyrolysis temperature had no significant effect to the retention value (Table 3). Further analysis in Table 4 showed that D. latiflorus Munro vinegar had higher retention value and significant difference compared to B. balcooa Roxb. and G. robusta Kurz. The retention value of D. latiflorus Munro was 26.28 kg/m3 and 26.66 kg/m3, for 300°C and 400°C pyrolysis temperature, respectively. The retention value in this study was superior to previous study that investigated the retention value of preserved rubberwood in wood vinegar. They found the retention value of rubberwood was 20.81 kg/m3 (Vachlepi et al., 2015).
The efficacy of preservative to increase wood resistance to termite attack could be assessed by calculating termite mortality (Ngadianto et al., 2012). Table 2 depicts the analysis of variance of the application of Hevea brasiliensis preserved with bamboo vinegar. It shows that the treatment had significant effect on the mortality rate of subterranean termite (p<0.05). Nevertheless, the analysis confirms that pyrolysis temperature and the interaction of temperature and bamboo vinegar had no significant effect on mortality rate of subterranean termite (p>0.05).
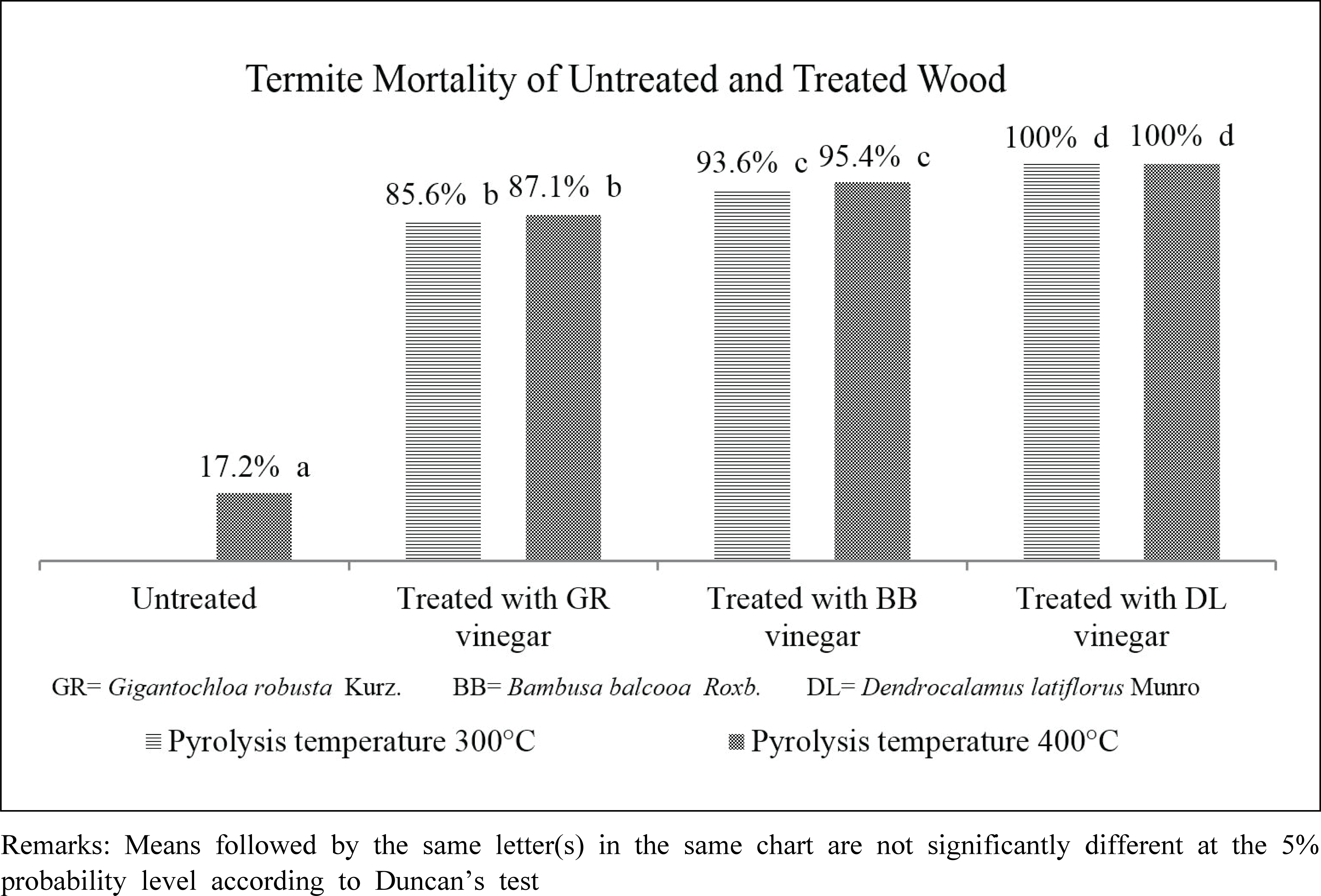
Bamboo vinegar from D. latiflorus Munro pyrolized at 300°C and 400°C exhibited strongest termiticidal activity compared to B. Balcooa Roxb. and G. Robusta Kurz (Fig. 1). The untreated/control wood, however, had the lowest termite mortality. This attests that the addition of bamboo vinegar as wood preservatives enhance the wood resistance against termite attack.
As presented in Table 1, total phenol and acid of D. latiflorus Munro bamboo vinegar were the highest compared to others, with the exception of total phenol at 300°C. The termite mortality rate was corresponding to the concentration of total phenol and acid in the bamboo vinegars.
Similarly, previous study showed that phenol or its highly oxygenated fractions was the largest component in the coconut shell oil that demonstrate superior anti termite activity against Odontotermes horni Wasmann, O. obesus Rambur, O. redamanni Wasmann, and Microtermes obesi Holmgren (Shiny and Remadewi, 2014). Comparable to this, wood vinegar made from Vitex pubescens Vahl that had a considerably total phenol and acid exhibited strong termiticidal activity against C. formosanus and R. speratus, at 10% and 5% concentration, respectively (Oramahi and Yoshimura, 2013). Acetic acid and other minor components were the largest component of wood vinegar made from Cinnamomum parthenoxylon which demonstrated remarkable termiticidal activity against Coptotermes curvignathus (Adfa et al., 2020). Additionally, the discrepancy of termiticidal activity in wood vinegar might be due to the organic fraction and acetic acid of wood vinegar (Yatagai et al., 2002). Phenolic compound could be attributed to the toxicity of insect digestion (Abbas et al., 2013).
According to the analysis of variance, bamboo vinegar, pyrolysis temperature and the interaction of both factors had significant effect on weight loss (Table 3). Even though both factors were significantly affected termite feeding rate, the interaction of bamboo vinegar and pyrolysis temperature were not significant.
| Source | Wood weight loss | Termite feeding rate | Retention of bamboo vinegar |
|---|---|---|---|
| Bamboo vinegar | ** | ** | ** |
| Pyrolysis temperature | ** | ** | ns |
| Interaction | ** | ns | ns |
Table 4 tabulated the results of weight loss of wood samples, wood resistance class, and termite feeding rate. According to Table 4, untreated wood showed poor wood resistance as it had the greatest weight loss (24.811%) and feeding rate (0.085 mg/termite/day). Rubber wood treated with D. latiflorus Munro bamboo vinegar had the lowest percentage of weight loss (6.144% and 5.012%) and termite feeding rate (0.037 mg/termite/day and 0.031 mg/termite/day) at 300°C and 400°C compared to other treatment.
The lowest feeding rate and weight loss in rubber wood treated with D. latiflorus Munro bamboo vinegar (Table 4) were also coupled with the lowest termite mortality (Fig. 1). Moreover, similar circumstance was also found in rubber wood treated with G.robusta Kurz and B. balcoa Roxb. bamboo vinegars. The untreated rubber wood had the highest termite feeding rate compared to other treated woods, 0.085 mg/termite/ day. This suggests that the wood preserved with bamboo vinegar was least preferable than the untreated wood due to the chemical compounds in bamboo vinegar. Termite feeding rate and wood weight loss of the untreated rubber wood in this study was slightly higher than that of found by Arinana et al. (2011). They found that termite feeding rate and wood weight loss of rubber wood were around 22% and 0.079 mg/termite/day, respectively. Hadi et al. (2014) argued that termite feeding rate was influenced by the existing environment, feed availability and other termite survival factors. Additionally, Subekti et al. (2018) confirmed that borax and wood vinegar preservation could reduce termite feeding rate. Their study found that the feeding rate of subterranean termite of P. merkusii was 1.5 mg/termite/day, 1.63 mg/termite/ day and 1.4 mg/termite/day for untreated, bamboo vinegar and borax preservation, respectively (Subekti et al., 2018).
According to SNI 7207 (2014), untreated wood was classified as very poorly resistant (class V) while treated wood classified as resistant (class II). It could be inferred that the addition of bamboo vinegar as wood preservative enhanced the resistance of rubber wood.
4. CONCLUSION
Production of phenolic and acid compounds in bamboo vinegars was highest at 400°C with the exception of total acid in D. latiflorus Munro. Bamboo vinegar pyrolized at 400°C could be beneficial in preventing Coptotermes curvignathus attack. Further study is required to determine chemical characteristics of bamboo vinegar.