1. INTRODUCTION
Living stands in pine forest are the main source of a wide range of biogenic volatile organic compounds (VOCs) in the forest atmosphere. It is well known that plant VOCs have various direct and indirect defensive roles against various abiotic and biotic stresses, and show various biological activities and have been used as alternative treatments for diseases (Ahn et al., 2018; Jeong et al., 2017). Most VOCs from trees contribute to the characteristic atmospheric environment of forests and provide useful bioresources of essential oil for the flavor and fragrance industries (Kim et al., 2017; Yang et al., 2019; Venkatesan et al., 2020).
In pine trees, the VOCs are produced mainly in needle tissue via photosynthetic pathways, and therefore living intact needles are important for continuous emission and accumulation of VOCs. However, needle abscission or senescence of living stands is usually caused by environmental stressors, such as drought or flooding, atmospheric temperature fluctuation, soil salinity, heavy metals, etc. (Finkelstein, 2013). The premature senescence and shedding of leaves can be induced by a deficit of water. The loss of pine needles during droughts involves true abscission; in other plants, the leaves merely wither and die. As temperatures decline in fall, the leaves of trees stop producing chlorophyll, and some species that contain large amounts of carbohydrates and possess the hereditary potential to do so begin to form anthocyanins in their leaves. As chlorophyll synthesis stops, the chlorophyll already present in the leaves begins to degrade and the newly formed anthocyanins are unmasked (Krammer and Kozlowski, 1979).
On the other hand, there are only a few studies whether the VOCs composition in needle are preserved or changed after needle abscission or senescence. The shed of aged or damaged needles in pine stands is important for the release of VOCs from the forest floor (Isidorov et al., 2003, Wang et al., 2018). Even when senescent or dead, pine needles contain high levels of terpenes and other precursors of the oxidized volatile compounds, formed as a result of enzymatic reactions (Isidorov et al., 2010; Jo and Kim, 2010; Zhang et al., 2018). Coniferous litter is also a good source of secondary metabolites that are released during chemical or biological decomposition processes. Intact Scots pine needles contain more than 70 organic compounds of different classes (Isidorov et al., 2003), as shown by solid-phase microextraction (SPME) and gas chromatography-mass spectrometry (GC-MS). They observed that the storage for four weeks on a conical floor stressed needles and caused their chemical compositions to vary.
It is obvious that volatile metabolomic patterns vary between intact and damaged or senescent needles, and statistical analysis should show which compounds constitute important discriminant variables. Besides the authentication and identification of needles, both marked and slight differences between the unique characteristics of needles can be studied with multivariate analysis. A practical and efficient assessment of biomass availability on pine forest floors could be made possible and easily accessible via the metabolomic interpretation of chemical components.
The objectives of the present study were to determine which VOCs in red pine needles change over the course of senescence or after shedding in relation to chlorophyll variation in the needles, and hence to reveal similarities and dissimilarities among intact, senescent, and litter needles.
2. MATERIALS and METHODS
The needles of red pines (Pinus densiflora Sieb. et Zucc.) were collected from standing trees at three site at Seoul National University, Seoul Campus, Republic of Korea (37°27'28.0"N 126°56'56.5"E) in the period of from November 2019 until January 2020. Intact needles were collected with an amount of 5-10 g for each stand and combined as a sample set, and senescent and litter needles were collected from broken branches and the upper layer of fallen needle on the wide-spread floor zone under the tree stands, respectively. Information on the samples is presented in Table 1, and sample code was named to reflect sampling site position and date. Collected needles (50-100 g in a sample set) were stored at -80°C after collection for further analysis. The experiments were repeated three times by pooling samples.
Frozen needles were crushed and milled in liquid nitrogen using a Freezer/Mill cryogenic grinder (SPEX 6875D, SPEX Co., USA) to avoid any chemical decomposition or change in the chemical composition of the needles after collection. Some of the milled needle samples were further freeze-dried and used for pigment analysis.
The chlorophyll a (Ca), chlorophyll b (Cb), and carotenoid (Cx-c) contents of the samples were determined according to the method described by Wellburn (1994). Briefly, 1 g sample mixed with 30 mL methanol in a 50 mL conical tube (polypropylene) was sonicated for 1 h and then shaken for 1 h. Samples were filtered with a 0.2 μm syringe filter. Absorbance was read at 666, 653, and 470 nm on a spectrophotometer (SpectraMax® ABS Plus, Molecular Devices LLC., USA) to determine Ca, Cb, and Cx-c content, respectively. The experiments were repeated three times. The amount of these pigments in the samples was calculated according to the formulae of Lichtenthaler and Wellburn (1983) as shown in the following equations, and expressed in microgram per unit dry weight gram of sample.
where, A666, A653, and A470 are absorption values at 666 nm, 653 nm, and 470 nm spectral wavelength, respectively.
Next, the composition of VOCs emitted from the freeze-milled samples was investigated using a headspace- solid phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC/MS) system (TSQ 8000, Thermo Fisher Scientific Inc., USA). Five hundred milligram samples were placed into 25 mL glass vessels and 4 μL of internal standard solution (100 ppm 1,4-dichlorobenzene-d4 in methanol) was added. The necks of the vessels were tightly sealed with an elastic Teflon film. After incubation on an autosampler at 30°C for 10 min, the VOCs from the headspace were adsorbed on an SPME fiber coated with divinylbenzene/polydimethylsiloxane (DVB/PDMS, 50/30 mm, 10 mm fiber length, SUPELCO Co., USA). The total exposition time was 40 min and every 12 s the vessels with needle samples were slightly shaken to mix the gas phase for 10 s. After adsorption of the headspace, VOCs were desorbed by introducing the SPME fiber into the injector of the gas chromatograph with mass spectrometer. The injector temperature was set at 250°C. During injection, the fiber was maintained for 2 min in the injector, which was used in split mode (10:1). Components were separated on a DB-wax fused silica column (60 m length × 0.25 mm internal diameter × 0.5 μm film thickness). The carrier gas was helium at a flow rate of 2.0 mL/min. The oven temperature was altered as follows: the thermostat temperature was initially 40°C (holding time 2 min), then was increased to 150°C (holding time 10 min) at a rate of 4°C/min, then to 200°C (holding time 5 min) at a rate of 3°C/min, and finally to 240°C (holding time 5 min) at a rate of 10°C/min. The mass spectrometer transfer line temperature was 250°C and the ion source temperature was 250°C. Mass scanning was performed from 35 to 550 amu for with scan time of 0.2 s. Component qualification was conducted by comparison of the measured mass spectra with those from an NIST/EPA/NIH Mass Spectral Library (version 2.0 g, NIST, USA) and additional Kovats retention index information (the retention times of alkanes were obtained by direct injection of standard solution under the same GC-MS conditions). To calculate retention time index, a standard solution containing C7-C30 normal alkanes in dichloromethane was used. The area of corresponding peaks was calculated by integration of peak intensity on the selected ion monitoring chromatogram from the total ion chromatogram (the parameter is shown in Table 3). The calculated area of each compound was normalized by dividing by the internal compound peak area and multiplying by 100 to give the semi-quantitative composition of VOCs. The two-array sample data sets were used for the following statistical analysis.
Principal component analysis (PCA) was implemented on the two-way array to overview data structure and relation trend between data sets. Results were presented as score plots. For further discriminant analysis, the orthogonal partial least square discrimination analysis (OPLS-DA) was conducted. OPLS-DA model is a useful discriminant model for metabolic fingerprinting studies of chromatography data sets (Bernal et al., 2016). An OPLS-DA model was therefore constructed for classification of the samples with chlorophyll variables to determine the important compounds among samples. The PCA and OPLS-DA models were created using SIMCA 15 software (Umetrics Inc.), and bivariate correlation analyses were conducted using IBM SPSS Statistics (IBM Co., ver 25.0).
3. RESULTS and DISCUSSION
The amounts of Ca, Cb and Cx-c in needle samples are shown in Table 2. The average amount of both Ca and Cb was lowest in litter needles and highest in intact needles, while there was no obvious trend or pattern for Cx-c. During the growing season, chlorophyll is constantly broken down and replaced. According to Taylor and Whitelaw (2001), abscission is frequently associated with senescence as both processes are initiated in evergreen plants by many of the same developmental as well as environmental and stress factors, such as changed photoperiod and temperature. Abscission involves the shedding of aged or diseased organs and often occurs as a consequence of senescence related to the death of an organism or some part of it. The low efficiency of photosynthesis below a certain threshold causes the senescence of needles and abscission following cessation of photosynthesis and an endogenous decline in plant hormones (Thimann, 1985). As the photoperiod decreases in the fall, the synthesis of new chlorophyll is prevented by an abscission layer of cork-like material that forms due to the rapid division of cells near the leaf stem (Webster and Leopold, 1972). This abscission layer prevents transport of carbohydrates and minerals to and from leaf, causing chlorophyll to be broken down but not replaced. Therefore, a lower amount of Ca and Cb in needles is related to senescence or abscission, although this relationship is not straightforward because environmental and stress factors can also influence Ca and Cb levels.
In the present study, the Ca/Cb ratio was 1.7 ± 0.1 in intact green needles, 1.0 ± 0.1 in senescent yellowish- green needles, and 0.7 ± 0.1 in brown litter needles, as shown in Table 2. The Ca/Cb ratio was high in intact needles and low in litter needles because in many species Ca is destroyed more rapidly than Cb in the fall and under water stress (Krammer and Kozlowski, 1979). Therefore, the Ca/Cb ratio was regarded as a good indicator of three levels of senescence (intact, senescent, litter), whereas Ca, Cb, and Cx-c values appear to be poor indicators of senescence as they varied greatly within the same needle sample. Therefore, the Ca/Cb ratio was used to investigate variation in VOCs among needle samples.
HS-SPME-GC/MS analysis is a powerful tool for analyzing the metabolomic patterns of essential oils (Goncalves et al., 2012). Table 3, 4, and 5 show the qualitative and semi-quantitative (quantitation was performed using an internal normalization method) composition of the VOCs emitted from the needles in the present study. Each component was identified based on its mass spectrum using a NIST library search program (NIST MS Search 2.0 g). Interestingly, the VOCs content of the senescent and litter needles was comparable to that of intact needles, but the chemical composition of the VOCs was slightly different.
Both non-terpenic aliphatic compounds and terpenic compounds were found in all needles (Table 4 and 5). The most abundant chemical group consists of terpene compounds: monoterpenes and sesquiterpenes. The major volatile compounds emitted by intact needles were an alkene (2-hexenal), monoterpenes (α-pinene, β-myrcene, β-phellandrene, myrcene, 2-β-pinene, d-L-limonene, L-bornyl acetate, and camphene), and sesquiterpenes (caryophyllene and germacrene-D). These results are similar to those of Lee et al. (2005) and Yu et al. (2004).
The VOCs composition emitted from senescent and litter needles was similar to that of intact needles, but the emissions of germacrene-D, 2-hexenal, and L-bornyl acetate were much lower in the senescent and litter needles. Several alkanes, such as a C6 compound of 2-hexanol, C5 compounds of 4-methyl-2-pentanol, 4-methyl-2-pentanone, and 2-methyl-3-pentanone, and a C4 compound of 2-butoxyethanol, were detected only in the senescent and litter needles, in addition to terpenic compounds, such as linalool oxide, myrtenal, pinocarveol, p-cymene, p-cymenen, caryophyllene oxide, and humulene oxide. According to Isidorov et al. (2003), oxidized or modified forms of terpenes were increased or found only in the litter needles of the Scots pine, including toluene, p-cymene, verbenene, p-cymenen, p-cymene-8-ol, anisaldehyde, and linalool acetate as minor compounds. The authors suggested that most of these compounds are secondary products of microorganism metabolism involving dehydration and oxidation of precursor terpenic compounds in intact needles. Our results are similar to those of Isidorov et al. (2003), but the experimental evidence on this topic is insufficient and further study is needed.
As shown in Table 4 and Table 5, major compounds with higher ratio values are more important than minor compounds with lower ratio values. However, the more compounds in a sample, the more complex and difficult it becomes to determine which compounds differ between levels of senescence. Therefore, we used a clustering and correlation statistical technique to determine which compounds among the VOCs of needles are most important for identifying senescence, and then to investigate changes in VOCs during senescence or after shedding.
In order to improve the quality of the data by reducing inconsistency and noise, pre-processing algorithms were applied to the raw data. To minimize data skewness, the log transformation (log x) was applied to the raw data in Table 4 and Table 5, and then Pareto scaling was applied to eliminate the unfavorable effects among compounds present in substantially different amounts.
PCA was applied to the pre-processed data to investigate the correlation structure.
On the PCA-derived score plot (Fig. 1A), intact needles were located far from senescent and litter needles, indicating that intact needles are different based on principal component (PC) 1. The first two PCs were able to explain 56.7% of the total variance of the variable set, and there was no outlier in this model. However, there was overlap between senescent and litter needles; therefore, it was not possible to differentiate these two variables on this PC1 and PC2 plane model. While chlorophyll content variation was high among samples due to the ecological environment (Shan et al., 1996), seasonal variation (Gond et al., 1999; Deligoz et al., 2018), soil type, etc., the Ca/Cb ratio has lower deviation in the same sampling part and was significantly different between samples. Therefore, the Ca/Cb ratio was used to define classes in a PCA-derived score plot (Fig. 1B) with three classes (Ca/Cb ratio values: Class 1, 0.5–0.95; Class 2, 0.95–1.35; Class 3, 1.35–1.80). The senescent and litter needles were more easily differentiated based on Ca/Cb ratio; Y31, Y32, and Y33 were close to the litter needles (Class 1) due to their low Ca content (Table 2). Thus, it is thought that the Ca/Cb ratio value is an ideal discriminant variable for intact and litter needles on the PC1 plane of a PCA-derived score plot.
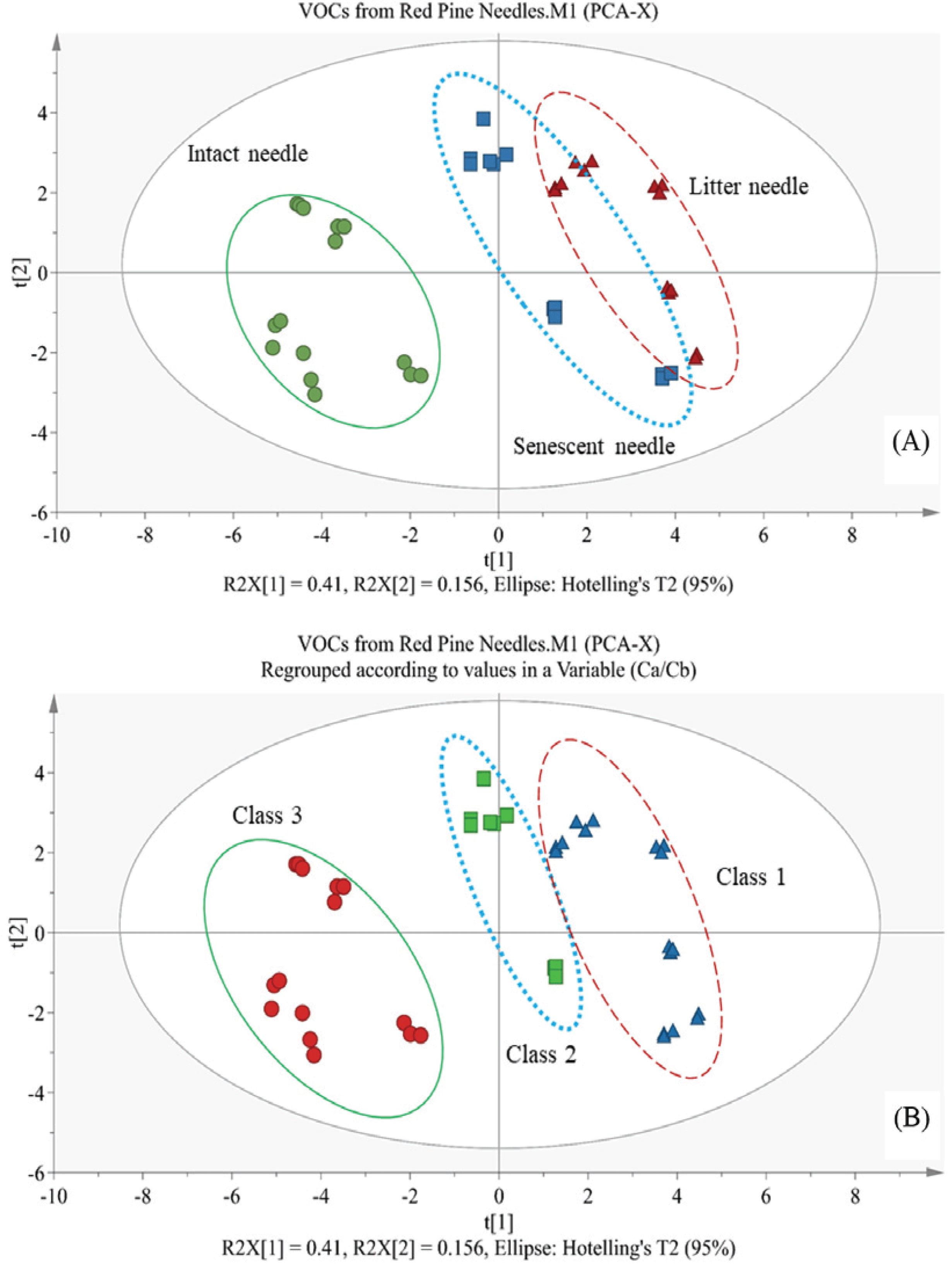
The OPLS-DA model was applied to the data set after reprocessing according to Ca/Cb ratio classes in order to investigate variability in chemical compounds to discriminate between senescence levels in needles.
The resulting score plot (Fig. 2A) shows that classification is possible based on Ca/Cb ratio classes. The plot (Fig. 2B) strongly indicates that the original model is valid; all predicted Q2-values are lower than the original points and the regression line of the Q2-points intersects the vertical axis below zero. Also, all R2-values to the left are lower than the original points to the right, which is another indication of the validity of the original model.
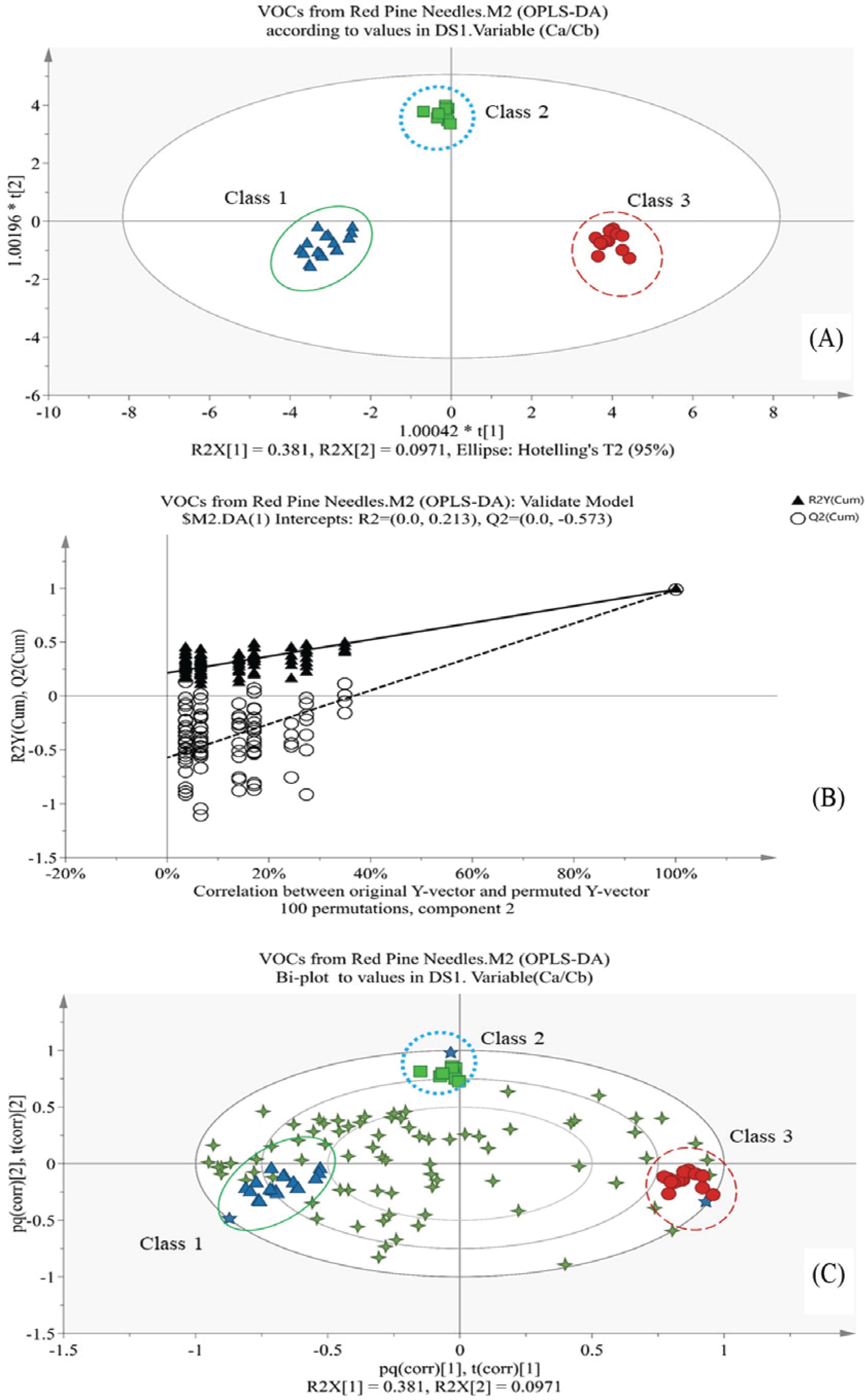
The variable importance for the projection (VIP) plot (Fig. 3) summarizes the importance of the variables both to explain variables (compounds) and to correlate to sample character in a statistical projected plane. The non-terpenic compounds (E)-2-hexenal, 1-penten-3-ol, 4-methyl-2-pentanone, 2-ethyl furan, (Z)-3-hexen-1-ol, and 2-penten-1-ol, and terpenic compounds of camphor, L-bornyl acetate, myrtenol and caryophyllene oxide were importantly found (VIP value > 1.0) in the VIP plot, and deviated more among needles than the terpenic compounds. Most strong (VIP value > 1.5) VIPs were camphor and 1-penten- 3-ol, without 2-hexenal due to its large deviation, and were thought to have the critical role in the overall discrimination in the OPLS-DA-derived score plot, especially to explain the differences between intact and litter samples. And figure 2C shows the distribution of VOCs related to sample classification; their distribution deviated among needle samples. The contribution list (Table 6) from the bi-plot (Fig. 2C) shows the difference between each point (scaled and centered as the workset) and the average of the model, which indicates the characteristics of each class and which compounds are discriminants. For the intact needles (Class 1), 1-penten-3-ol and 2-ethyl furan, and L-bornyl acetate were the strong (score > 1.5) contributors to the dissimilarity of this class to the others, while camphor, pinocarvone, and myrtenol were for Class 3 (litter needles), and 2-heptanal, β-myrcene, 3-methyl-2-buten-1-ol, and spathulenol were for of Class 2 (senescent needles) in the OPLS-DA-derived score plot plane. 1-Penten-3-ol and camphor were most strong contributors for intact (Class 1) and litter (Class 3) needle, respectively, which result had a same trend with the result by VIP values evaluation.
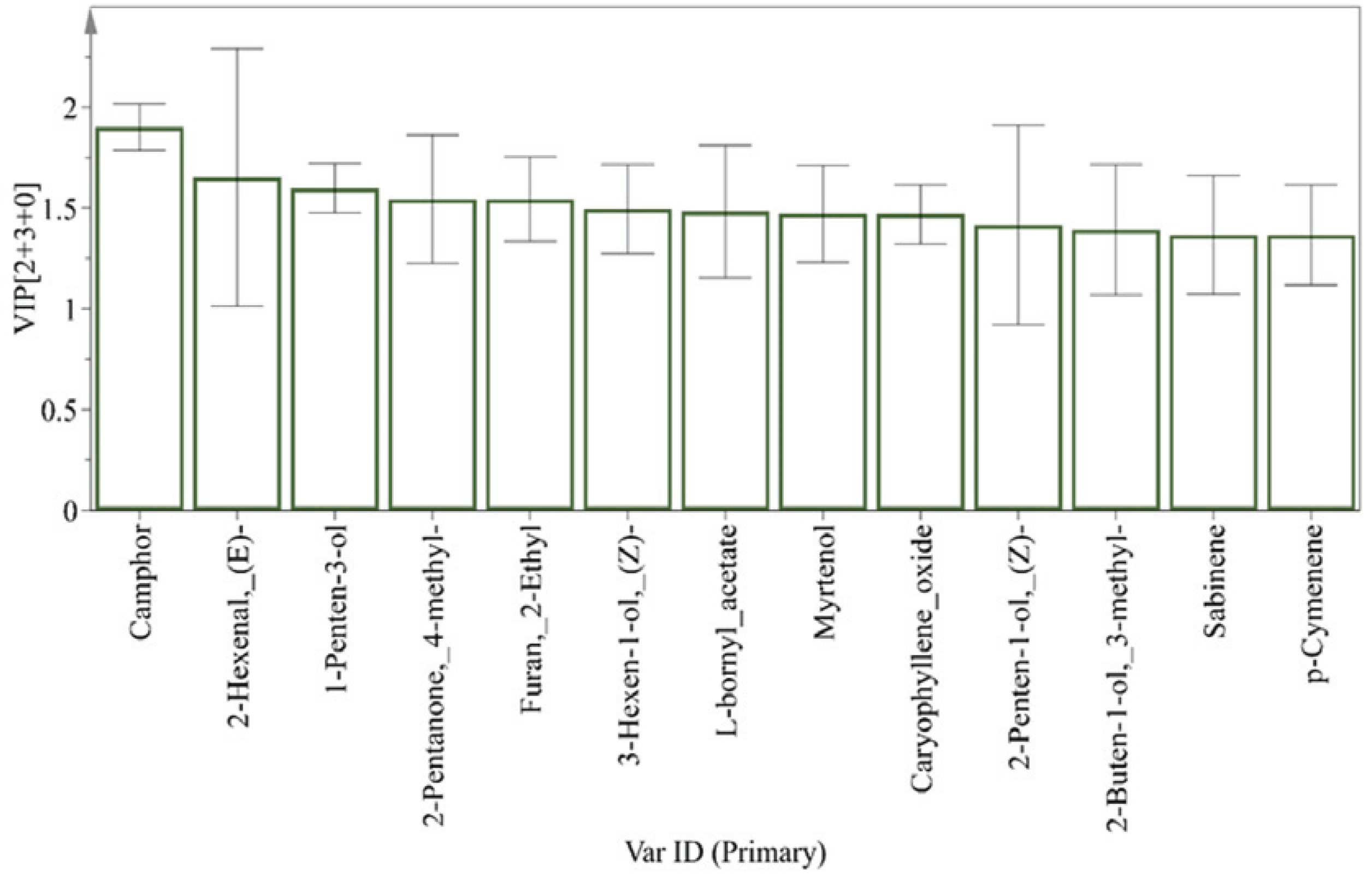
The content of the leaf aldehyde (E)-2-hexenal, an aroma-active compound of fresh red pine needles (Yu et al., 2004), was higher in intact needles and almost absent in litter needles (Table 4). The content of the leaf alcohol (Z)-3-hexen-1-ol followed a similar pattern to (E)-2-hexenal. The C6 volatile compounds (Z)-3-hexen-1-ol and (E)-2-hexenal are biosynthesized via the lipoxygenase pathway, which includes a secondary hydroxyperoxide lyase reaction from unsaturated fatty acids, such as linolenic acid and linoleic acid, and the formation of the C5 volatile compounds 1-penten-3-ol and 2-penten-1-ol from inhibited secondary hydroxyperoxide lyase (Shen et al., 2014). The levels of (E)-2-hexenal, 1-penten-3-ol, (Z)-3-hexen-1-ol, and 2-penten-1-ol were significantly higher in intact needles than in senescent and litter needles, and these compounds with high VIP values were characteristic non-terpenic compounds of intact needles. As for terpenic compounds, also shown in Table 4 and Table 5, the content of camphor, an oxidized form of monoterpene with a borane skeleton, was higher in senescent and litter needles than in intact needles, while the opposite was true of L-bornyl acetate, which features the same borane skeleton. Furthermore, the levels of p-cymenene and p-cymene, which are chemically stable compounds due to the presence of a benzene ring, were higher in litter than intact needles, while the content of labile bicyclogermacrene and germacrene- D were higher in intact needles. Oxidized or modified terpenes were contributors (Table 6) and had high VIP values (Fig. 3) in litter needles in the OPLS-DA model, and were characteristic compounds of senescent and litter needles.
Therefore, it was suggested that the oxidation reaction involving the lipoxygenase pathway occurred in the intact needles, to a lesser extent or not at all in the senescent and litter needles, and that the terpenic compounds were also degraded or modified during senescence or after shedding. Unfortunately, in this paper, there were no experimental data on the evidence about oxidation, degradation, and modification reactions of VOCs in the process of senescence and shedding.
Variables of contribution list (Table 6) and VIP plot (Fig. 3), were further processed by a bivariate regression with Ca/Cb ratio with no data transformation, to find out which compound has more higher correlation with Ca/Cb ratio, meaning the dependence on degree of senescence or after shedding.
As shown in Table 7, with the exception of β -myrcene, all variable revealed significant (p > 0.01) correlation coefficients with a sign of positive or negative. The level of variables of positive correlation coefficients increase dependently on Ca/Cb ratio increase, which reflects the closeness to being intact, while for negative correlation Ca/Cb ratio dependently decreases which reflects the closeness to being litter.
1-Penten-3-ol had the highest positive correlation coefficient, while caryophyllene oxide had the negative one. Interestingly, this result informs of importance of 1-penten-3-ol as an excellent discriminant of intact needle, in that this is a strong contributor for intact needle and also important VIP on OPLS-DA evaluation. However, camphor, having strong contribution to litter needle and the highest VIP value, revealed relatively low negative correlation coefficient but significant. This result was thought to be derived by very low content of intact needle and high content of senescent and litter needles (Table 5) because resultantly data points deviated largely from linear regression line.
From the additional bivariate regression analysis, several compounds of VOCs in red pine needle increase or decrease proportionally to degree of senescence of needle, represented by the Ca/Cb ratio. And it was thought that these compounds react very sensitively and sequentially to surrounding environment changes during senescence or after shedding and would be useful as indicators or makers for degree of sensescence or aging after shedding.
4. CONCLUSION
The present study successfully determined which VOCs in red pine needles are changed over the course of senescence or after shedding by statistical analyses and finally revealed similarities and dissimilarities among intact, senescent, and litter needles. The Ca/Cb ratio was a good indicator of whether needle was intact or not; the intact needle had higher average Ca/Cb ratio than the senescent and the litter needles. Whether needles were intact, senescent, or litter was well explained by multivariate statistical interpretation of VOCs using the PCA and the OPLS-DA models with Ca/Cb ratio values as a classification variable.
Further investigation on effects of variables (chemical components) to needle classification revealed several significant compounds. 1-Penten-3-ol and camphor were most strong contributors for intact (Class 1) and litter (Class 3) needle, respectively, which result had a same trend with the result by VIP values evaluation. The oxidation reaction involving the lipoxygenase pathway occurred in the intact needles, to a lesser extent or not at all in the senescent and litter needles, and that the terpenic compounds were also degraded or modified during senescence or after shedding. The content of compounds having high correlation coefficient would increase or decrease proportionally to degree of senescence of needle, represented by the Ca/Cb ratio, indicating that these compounds react very sensitively and sequentially to surrounding environment changes during senescence or shedding.
In addition, this study suggests that senescent and litter needles along with intact needles are also good bioresources of biogenic volatiles in the forest ecosystem and fragrance and flavor industry because major compounds of VOCs were also found between needles, but their chemical composition or content of several chemical compounds are markedly different. It was thought that further works on biological or physiological or environmental pathway should be conducted to elucidate or explain the deviation of VOCs in needle samples.








