1. INTRODUCTION
Oil palm plantation is considered a well-known tropical plantation in Southeast Asia for the past two decades. This vegetative plant plays a major role in various strategic industrial projects as it can increase national income in various important sectors in palm oil-producing countries, such as Indonesia, Malaysia, and Thailand (Irvan et al., 2018). As stated by Prabuningrum et al. (2020) increasing the area and production of oil palm has an impact on increasing the waste from these plants when they are no longer productive at the age of more than 25 years. Palm oil produces 22-palm fronds per-year with an average weight of 2.20 kg fronds for each palm frond. These figures show the enormous potential of oil palm fronds. Due to large amounts of oil palm frond a produce in palm oil plantation, the selling price of oil palm fronds activated carbon can be reduced to a minimum. Besides, the sustainability of oil palm fronds activated carbon production can be maintained. However, the utilization of oil palm fronds is still constrained by low levels of digestibility due to high levels of neutral detergent fiber and lignin (Hamchara et al., 2018).
Oil palm fronds fall into the category of wet by-products, approximately contain 70–75% of water content (Pambayun et al., 2013; Ramdja et al., 2008) with production of oil palm frond approximating 5500 kg/ha/years (Hamchara et al., 2018). The availability of palm oil and its waste is abundant. This potential can be utilized by using palm oil waste as an alternative material in carbon manufacturing. This idea resulted in the creation of new economic value, thereby reducing palm oil waste. The above background leads to the importance of conducting research on the manufacture and characterization of activated carbon from oil palm fronds with Na2CO3 and NaCl as activators based on activated carbon quality standards according to SNI 06-3730-1995. The reason for choosing these activators because still a few researchers using Na2CO3 and NaCl as activating agents in the manufacture of activated carbon especially based on oil palm frond, besides, being easy to obtain, low price and environment friendly.
Activated carbon is an amorphous carbon that possesses a surface area ranging from 1000 to 1500 m2/g and has been treated with steam and heat (Rahman et al., 2018). This treatment allows activated carbon to exhibit very strong affinity to absorb various materials with large capability, that is, 25–100% of activated carbon weight. This affinity relates to the internal pore structure, causing the activated carbon to act as an adsorbent. Activated carbons show selective adsorption properties that depend on the size or volume of the pores and surface area.
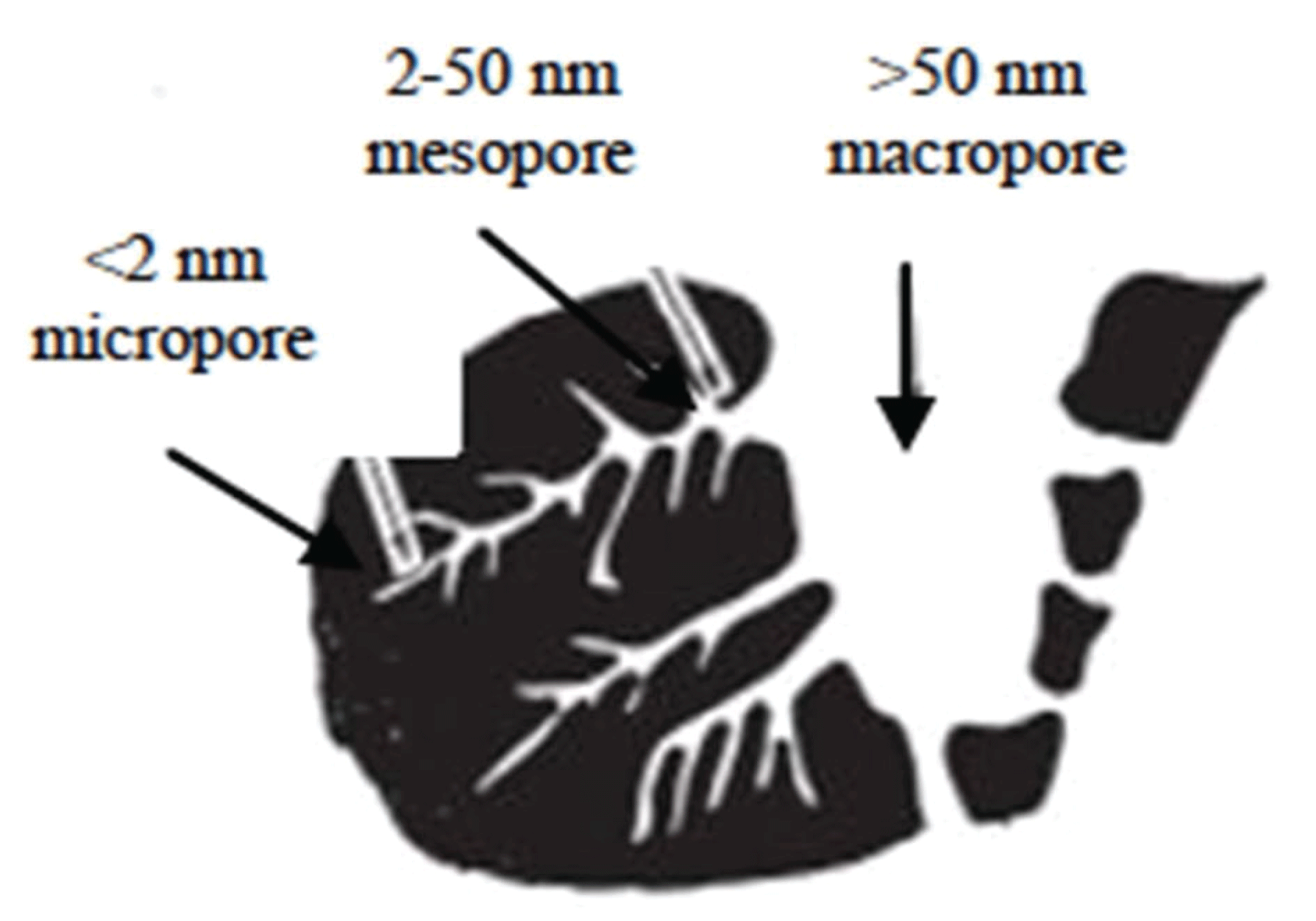
Activated carbon shows a wide range of utilization, in the food and drug industry, petroleum chemical industry, water purifier, shrimp cultivation, sugar industry, gas purification, catalyst, and fertilizer processing (Rahman et al., 2018). Activated carbon possesses a porous surface structure, which is classified by IUPAC as microporous, mesoporous, and macroporous. Fig. 1 shows a schematic of the active carbon pore structure.
Activated carbon is manufactured via two processes, namely, carbonization and activation. The activation process consists of two types, namely, physical activation and chemical activation. Physical activation by gas generally uses N2, CO2, and water vapor. On the other hand, chemical activation involves an activating agent that is generally a hydroxide (KOH or NaOH), ZnCl2, and H3PO4. Oil palm frond is expected to be a source of a raw material to produce activated carbon, besides coconut shells.Lignocellulosic material in oil palm fronds consist of 34.89% cellulose, 21.54% hemicellulose, 19.87% lignin (Maulina et al., 2018). Ash content in a charcoal as raw material was around 10-18% (Maulina and Anwari, 2019). Hong et al. (2012) stated that ash content of oil palm fronds approximately 12.30%.
2. MATERIALS and METHODS
The materials used in this study included of oil palm fronds, Na2CO3, NaCl, distilled water, iodine solution, sodium thiosulfate, and starch indicator.
The equipment used in this research included of furnace, digital balance, 32-mesh screen, porcelain cup, oven, pH meter, stopwatch, stir bar, tube jar, beaker glass, burette, dropper drop, thermometer, hot plate and magnetic stirrer, desiccator, Whatman filter paper, Fourier transform infrared spectrophotometer (FTIRShimadzu), and scanning electron microscope (SEM Evo MA 10 ZEISS).
Manufacturing of activated carbon is achieved through several processed from oil palm fronds: preparation, impregnation, carbonization, and analysis performance of activated carbon as a product.
Oil palm fronds were made into chips, oven-dried at 110 °C for 24 for hours, smoothed with a ball mill, and filtered using a 32-mesh sieve. Impregnation was done at 80°C for 2 hours, soaking at room temperature for 24 hours, using activator 5%, 7.5%, and 10% of Na2CO3 and NaCl. After impregnation, carbonization was conducted for 60 min at 400 °C, 500 °C, and 600 °C to produce activated carbon. The activated carbon was washed and filtered until pH 7, and then ovendried. Analysis of products included of yield, moisture content, ash content, volatile matter content, fixed carbon content, and absorption capacity of iodine (Iodine number).
Yield of activated carbon (Yac) aims to determine the amount of activated carbon produced after carbonisation and the activation process.
Yac = (Wac / Wopf) × 100%.
Remark: Wac: weight of activated carbon, Wopf: weight of oil palm fronds
Moisture content (MC) is the amount of water contained in activated carbon.
MC = [(W0 – W1) / W0] × 100%.
Remark:
W0 = Initial weight;
W1 = After ovendry weight;
Ash content = (ash weight / initial weight) × 100%.
Volatile matter content (VM) is volatile content
VM = [(W2 – W3)/ (W2 – W1)] × 100%.
Remark:
W1 = empty crucible weight + cover;
W2 = empty + sample + cover;
W3 = empty crucible weight + residue + cover
Fixed carbon content is the remaining carbon after carbonisation process and the activation process.
% Fixed carbon = 100% - (%volatile matter content - %ash content).
Absorption capacity of iodine (Iodine number) Iodine number is the mass of iodine in grams that is consumed by grams of chemical substances.
Iodine number (mg/g) = 10 [(Vb -Vt)/W2)] × W1 × Fp.
Remark: Vb: volume of blank titration; Vt: volume of sample titration; W1: 12.693 mg/ml; W2: sample weight (g); and Fp: dilution factor
Morphological analysis of activated carbon surfaces by using Scanning Electron Microscopy (SEM) with SEM micrographs magnification at 1000x. Fourier Transform Infrared (FTIR) was used to analyze functional groups of activated carbon. The spectra recorded in 45 scans per sample with spectrum range 4000 - 400 cm-1 and the resolution of 8.00 cm-1.
3. RESULTS and DISCUSSION
This study analyzed a yield of activated carbon and a number of characteristics of activated carbon, including the following: water content, ash content, volatile matter content, fixed carbon content, and iodine number. Sodium carbonate and sodium chloride activation mechanisms are:
Na2CO3 + 2C → 3 CO + 2Na, The presence of sodiumin the char leads to the oxidation of cross linking carbon atoms and form into slightly wrinkled or folded or puckered form.
NaCl + 2n H2O → Na+(H2O)n + Cl- (H2O)n, these molecules will be trapped in cellulose and cellulose become swelling.
Yield of activated carbon was measured after carbonization and oven dry step. Fig. 2 reveals the yield of activated carbon obtained by activation at various temperatures and concentrations.
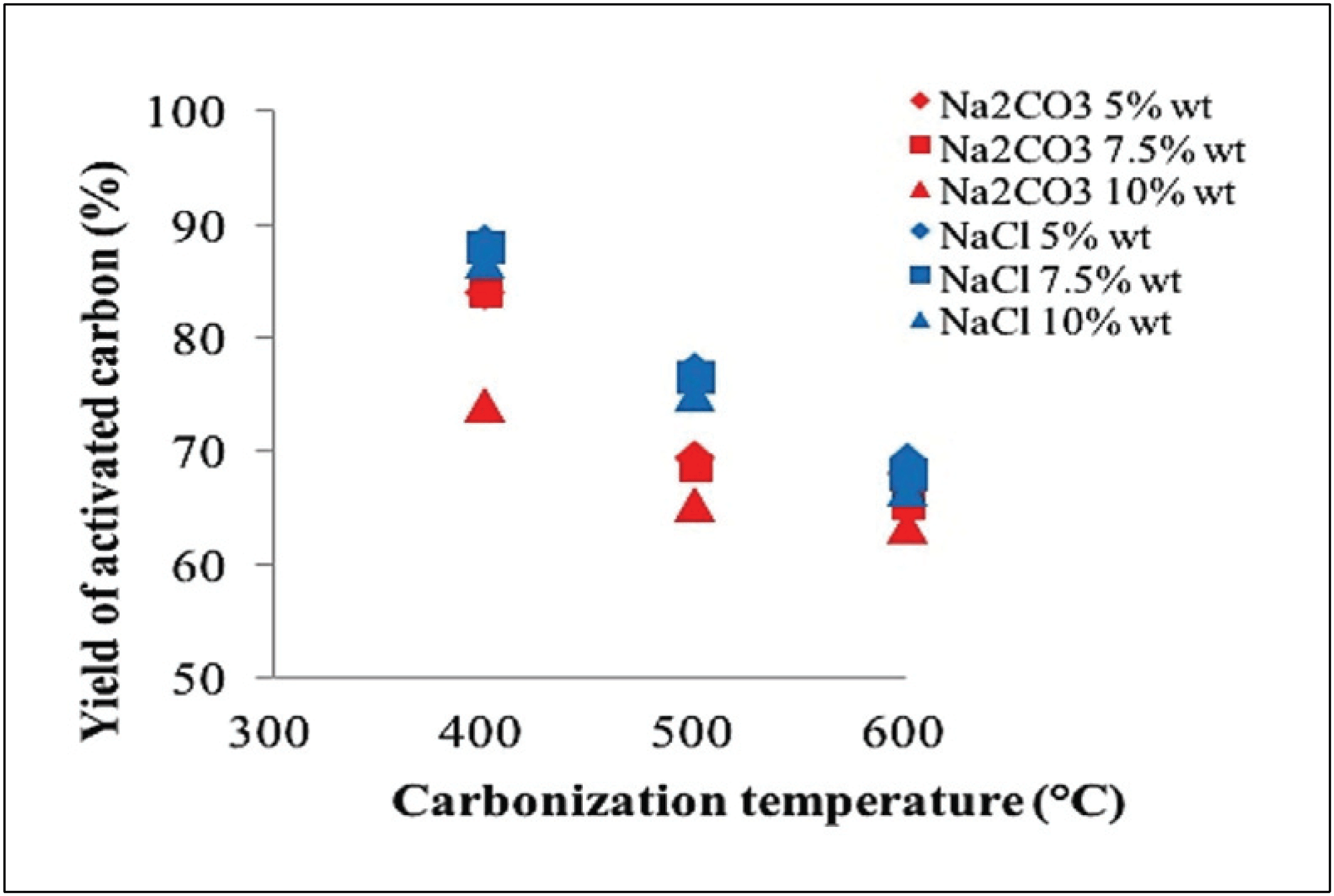
A total of 63.33–74.00% of activated carbon yield was produced using Na2CO3 activator, whereas 66.67– 88.67% of activated carbon yield was produced using NaCl activator. The yield of activated carbon from oil palm fronds decreased with increasing carbonization temperature of 400-600 °C. This result relates to the high rate of reaction carbon and activator substances to deliver volatile components along with increasing texture characteristics of the activated carbon, which forms pores (Chen et al., 2011) and carbon burning (Saka, 2012).
Activator concentration significantly influences the yield of the resulting carbon (Tan et al., 2008), where the amount decreases as the activator concentration increases. This phenomenon occurs because the activator will increase the release of volatile components due to dehydration reaction, which can increase carbon burning (Adinata et al., 2005).
The moisture content shows in Fig. 3 at various carbonization temperatures, activator types, and activator concentrations. The moisture content of activated carbon produced with Na2CO3 as activator reached 5.00-11.90%. The activator of NaCl produced moisture content of 4.90-13.80%. Moisturecontent decreased along with carbonization temperature due to the decreasing in the pores of activated carbon. This phenomenon results in reduced hygroscopic capability of activated carbon (Mitome et al., 2013). Moisture content in raw material lower than activated carbon, because of tar and organic materials are still inside the pore (Maulina and Anwari, 2019). Activator concentration also affects the moisture content produced. Increased water levels suggest that the activator degrades additional organic molecules during carbonization (Son et al., 2005). Fig. 3 reveals that the moisture content of the activated carbon higher with increasing activator concentration, promoting the dissolution of tar and organic minerals on activated carbon. Thus, the amount and depth of pores of activated carbon produced will higher with increasing activator concentration. Ultimately, this phenomenon will increase the hydroscopic characteristics to absorb water from air (Tani et al., 2014). Moisture content obtained were in accordance with the activated carbon quality standard based on SNI 06-3730-1995, which required a maximum water content of 15%.
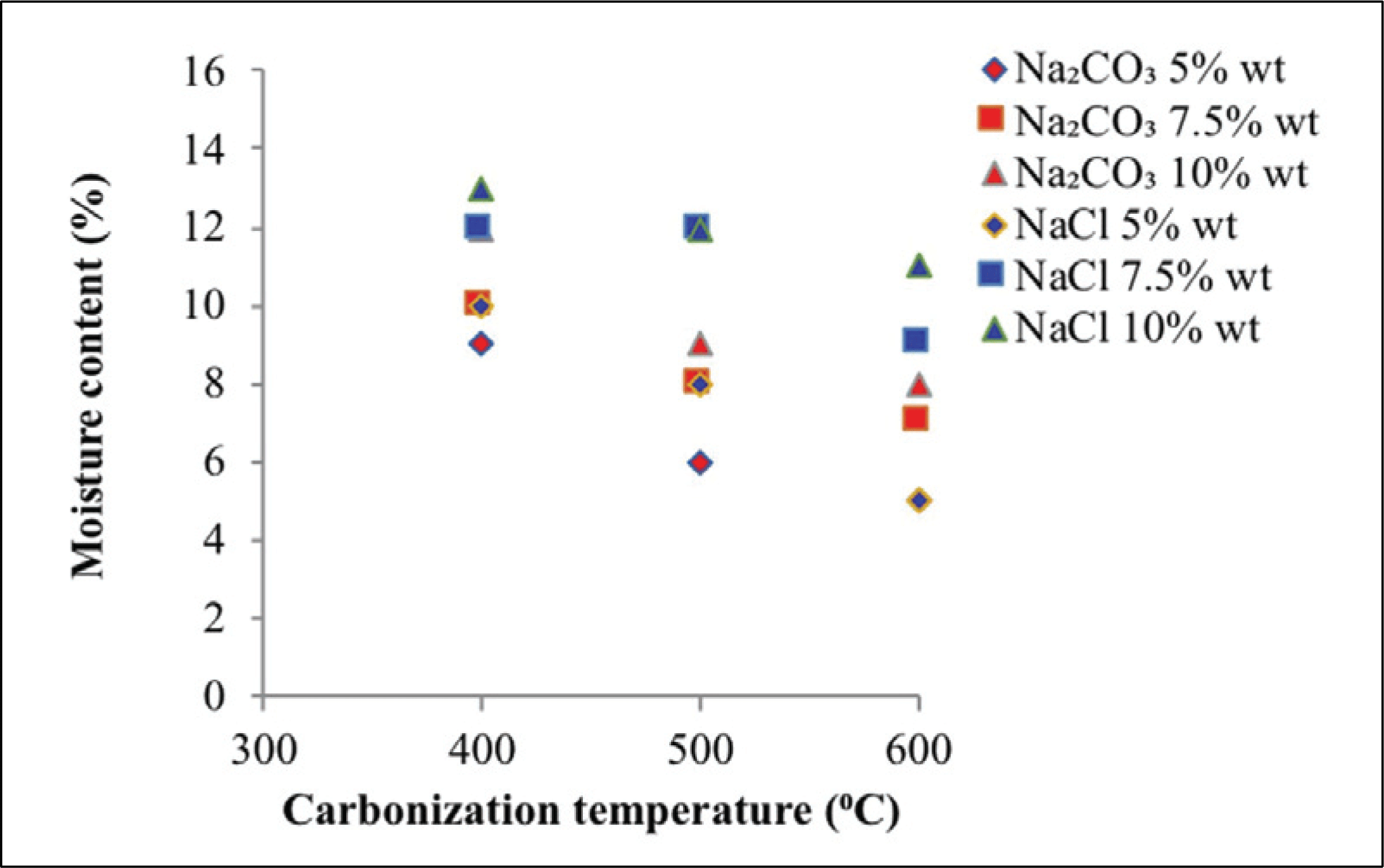
Moisture content analysis showed that sodium carbonate activators performed better than sodium chloride activators, that is, sodium carbonate activators are better than sodium chloride activators at dissolving tar and organic minerals upon impregnation and carbonization and will increase the number of pores, thus increasing water absorption from air.
Fig. 4 reveals the ash content at different carbonization temperatures, activator types, and activator concentrations in activated carbon.
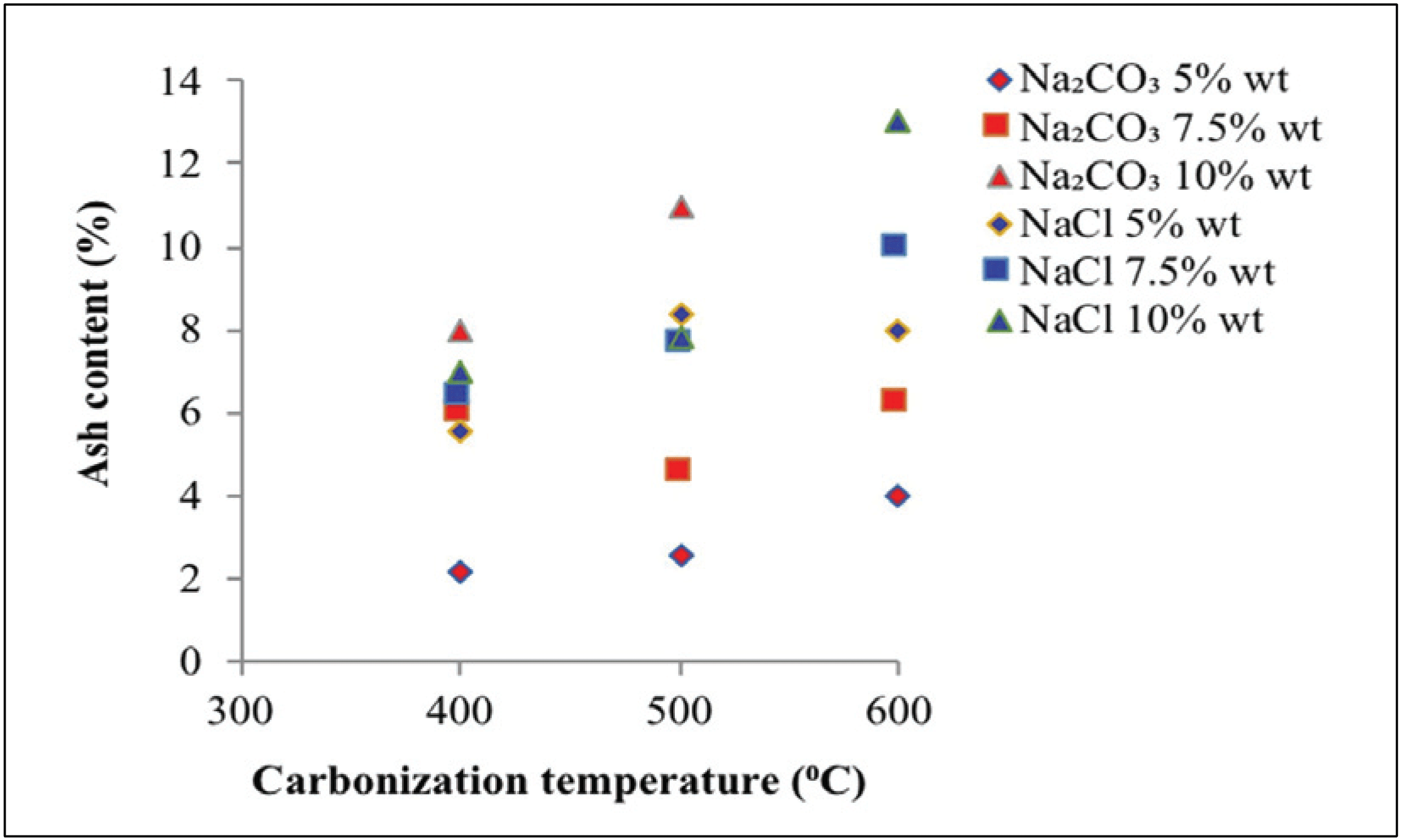
Fig. 4 shows that the ash content produced using Na2CO3 as activator reached 2.20–13.00%, whereas the activator NaCl produced an ash content of 5.60– 13.00%. Ash content in charcoal as a raw material higher than activated carbon, organic materials in raw material will generate metal oxides and will increase the amount of ash content (Maulina and Anwari, 2019). The ash content of activated carbon increased with increasing carbonization temperature. This finding indicates that an increase in carbonization temperature increases the vaporization of specific volatile compounds, which are within the particles at high carbonization temperatures (Rodenas et al., 2003). In addition, carbon burning will increase the ash content on activated carbon (Saka, 2012). Ash content increased along with the high concentration of activators. The high concentration of the activator will increase the surface area of activated carbon, which will result in formation of additional pores. Increased pores formation results in additional ash production (Tani et al., 2014).
High ash content can cause environmental problems in this case related to air pollution. As a solution to this problem, ash and silica content are used as reinforcement in rubber vulcanization (Sombatsompop, et al., 2004). Fly ash generated from desalination and power plants is used to capture CO2 emitted from industries (Alhamed et al., 2015).
Ash content is determined by the quantity of silica present in raw material. More silica content results in greater ash content (Kamariya et al., 2016). Silica can cause pore blockage in activated carbon, thus reducing the surface area (Jin et al., 2012). A high activator concentration will cause the silica to dissolve in the activator, thus increasing the surface area of activated carbon. Ultimately, this reaction increases pore formation (Tani et al., 2014). The resulted ash content using Na2CO3 activator was lower than that obtained with NaCl. This finding suggests that compared with NaCl, Na2CO3 as activator can better maintain heat in carbonization, thus preventing further oxidation against organic substances, which will yield undesirable substances such as ash (Son et al., 2005).
Fig. 5 shows the volatile matter content at various carbonization temperatures, types of activators, and activator concentrations in activated carbon.
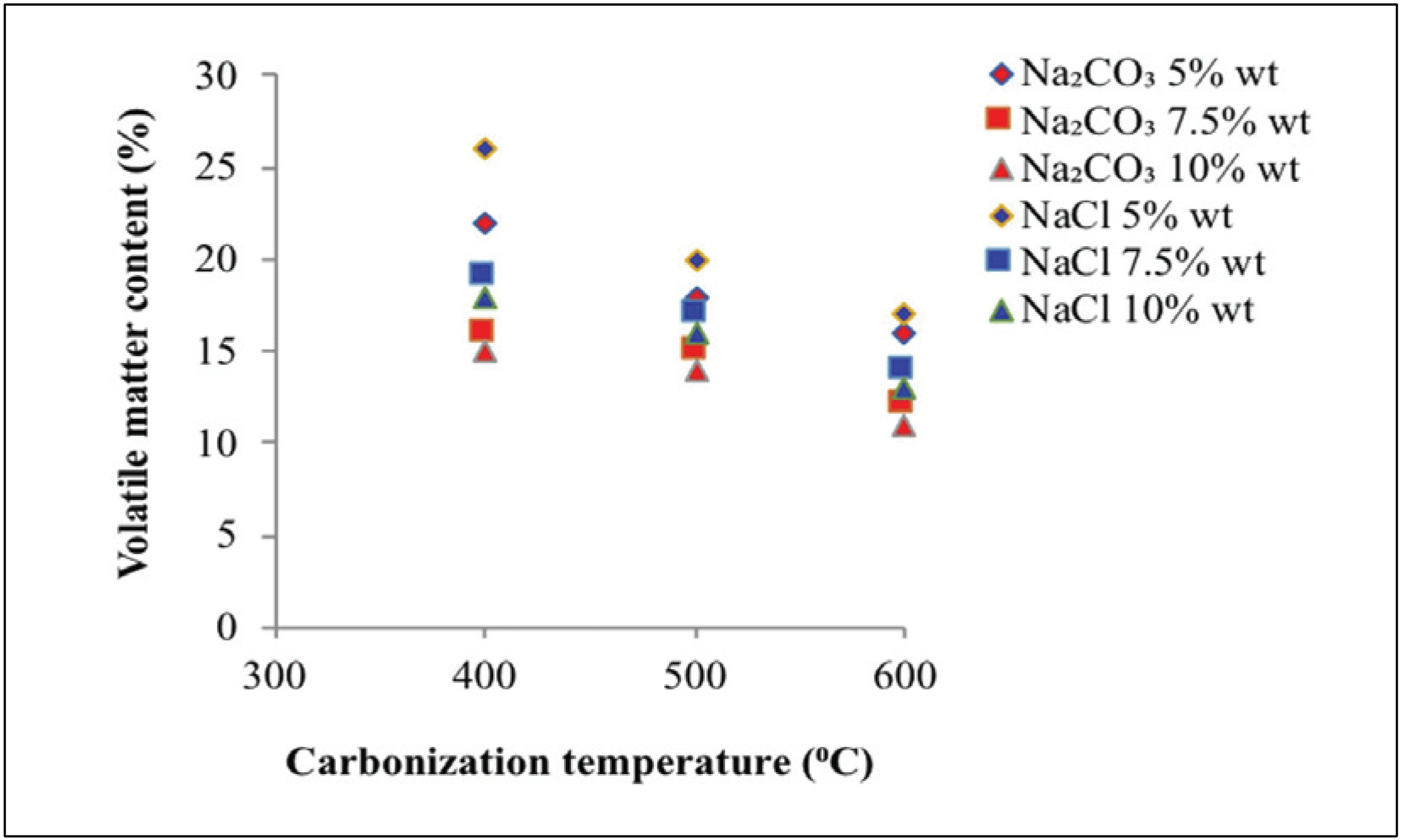
Fig. 5 shows that the volatile matter contents obtained with Na2CO3 and NaCl activators reached 11.00 –21.80% and 12.80–24.50%, respectively. Generally, volatile matter of raw material still higher compare to activated carbon, because of tar and material organic (Maulina and Anwari, 2019). The volatile matter content was decreased with rising the temperature of carbonization, that is, higher carbonization temperature indicates better decomposition of non-carbon compounds in activated carbon. This result indicates the production of volatile substances and carbon burning, resulting in low volatile matter content (Chen et al., 2011; Saka, 2012).
Fig. 5 also shows that an increase in activator concentration will decrease the volatile matter content. This finding is due to the added activator, which will seep, coat, and protect the material from heat. Thus, the high concentration of activators decreases the amount of ingredients burned (Caroline et al., 2015). The addition of the activator also caused the degradation of organic material, and this condition can weaken the surface structure of activated carbon and then cause the release of volatile substances and development of microporous structure on activated carbon. Na2CO3 activators are better than NaCl as Na2CO3 activators can produce lower volatile matter content. This finding is attributed to the capability of activator Na2CO3 to better seep, coat, and protect the material from heat than NaCl activator. Thus, a result of low volatile matter content was observed.
Fig. 6 reveals the fixed carbon content of activated carbon at various carbonization temperatures, types of activators, and activator concentrations.
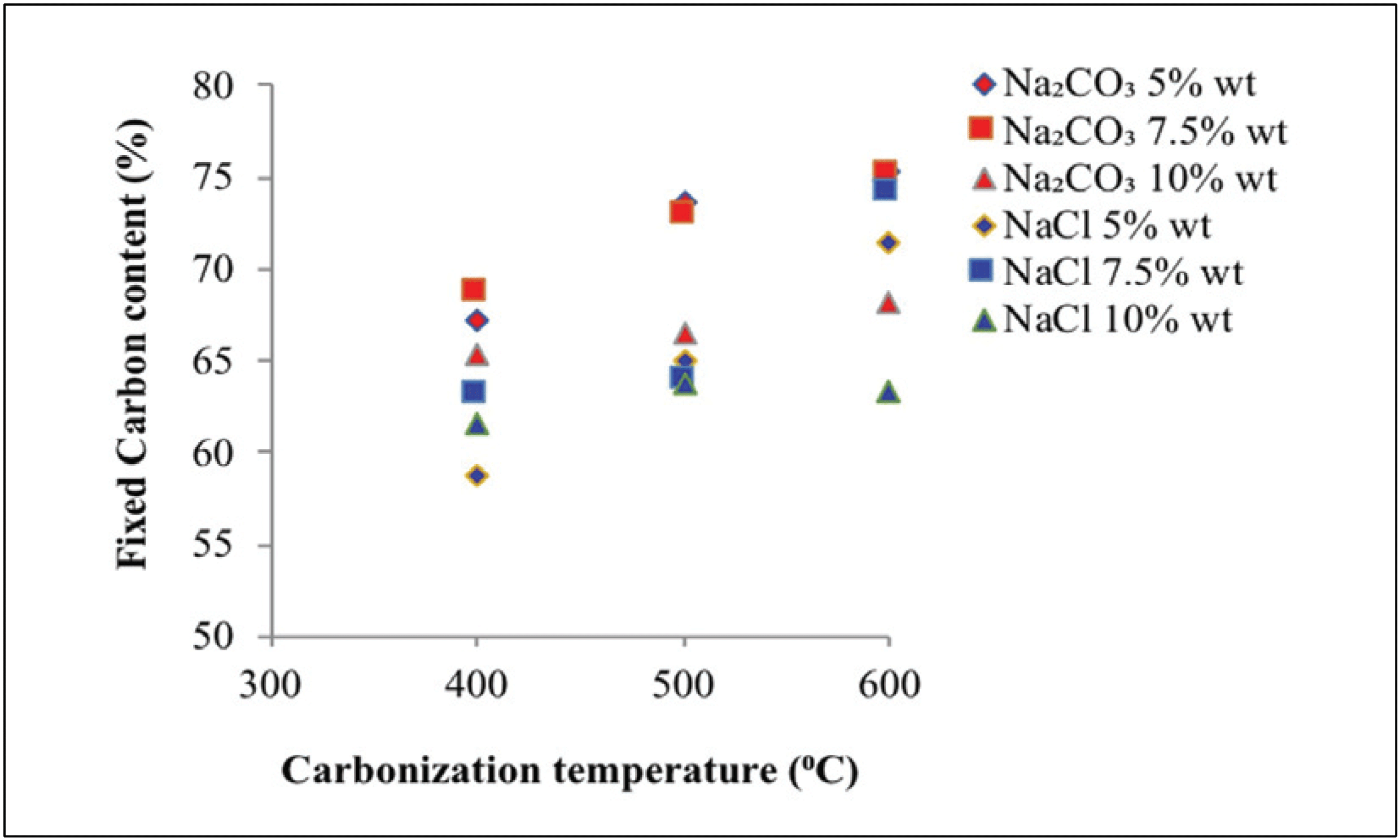
Fig. 6 reveals that the fixed carbon contents obtained using Na2CO3 and NaCl activators totaled 65.30 –75.30% and 58.70–74.20%, respectively. The fixed carbon content of activated carbon is inversely proportional to water, ash, and volatile matter contents (Caroline et al., 2015). In general, fixed carbon content rise with higher concentration of activator. The addition of activator concentration will increase the activated carbon content as the non-carbon compounds of carbonization will be more easily dissolved with the activator, as indicated by the dense color of the activated carbon (Caroline et al., 2015). Fixed carbon of raw material lower than activated carbon, because of organic material and tar content in raw material (Maulina and Anwari, 2019)
Fig. 6 also shows that 68.60% of fixed carbon content was obtained from sodium carbonate impregnation treatment at 400 °C with different activator concentrations. This level increased at 7.50% activator concentration and decreased to 65.30% at 10% activator concentration. Impregnation treatment of NaCl solution at the same temperature with different activator concentrations resulted in a fixed carbon content of 63.10%. This level increased at 7.50% of activator concentration and decreased to 61.60% at 10% of activator concentration. At least three factors affect the exchange in bound carbon content namely ash content, volatile matter content, and water content, of activated carbon (Tsoumis, 1991). In addition to the influence of ash and fly contents, the resulting fixed carbon content is also influenced by contents of lignocellulosic material as lignin and cellulose that could be converted to carbon atoms (Caroline et al., 2015).
Fig. 7 shows the adsorption capacity of iodine on activated carbon at various carbonization temperatures, types of activators, and activator concentrations. The iodine levels obtained with Na2CO3 and NaCl activators ranged between 482.34 and 888.55 mg/g and between 621.96 and 736.19 mg/g, respectively. Higher carbonization temperature indicated lower iodine absorption. This finding was attributed to two factors: breakdown of carbon surface due to the high temperature of carbonization and collapse of the wall due to carbon burning (Chen et al., 2011). Alkaline and salt activators can produce activated carbon with maximum micropores under operating conditions of less than 500 °C (Marsh and Reinoso, 2006).
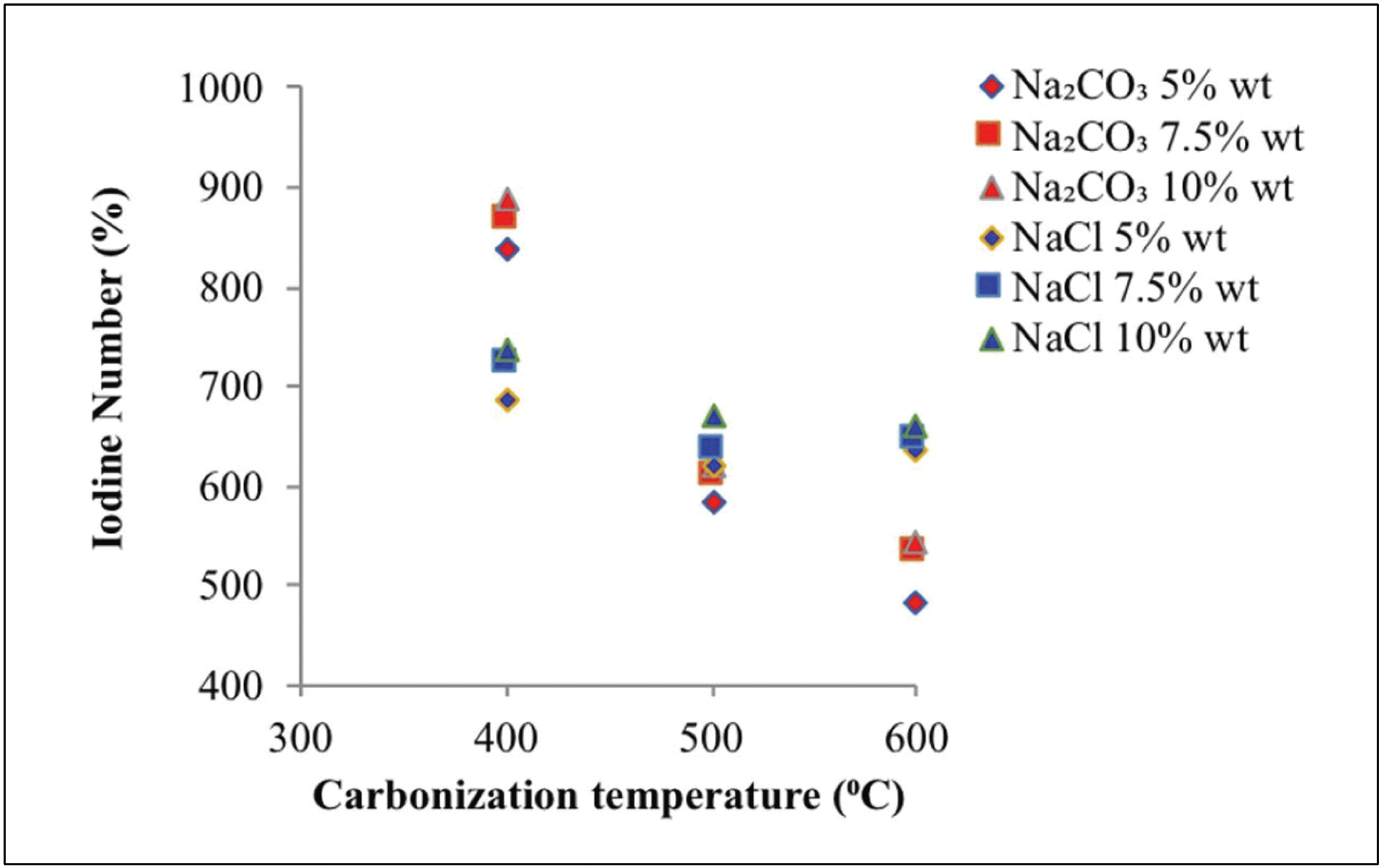
The volatile compounds present in the interior of activated carbonparticles will further evaporate at high carbonization temperatures (Rodenas et al., 2003). This reaction triggers carbon burning, which can increase ash content on activated carbon (Saka, 2012).
Increasing activator concentration increased the absorption power of iodine. This reaction is due to reduced tar levels observed along with increased activator concentration at the time of immersion. Thus, the number of pores that were present in the activated carbon increased, thus increasing absorption (Son et al., 2005). This finding is associated with a wide reaction between the activator substance and the carbon surface (Deng et al., 2010) resulting in an increased release of CO2 and CO gases, which triggered an increase in pore formation (Mitome et al., 2013).
The SEM analysis of active surface carbon shows on Figs. 8 and 9.
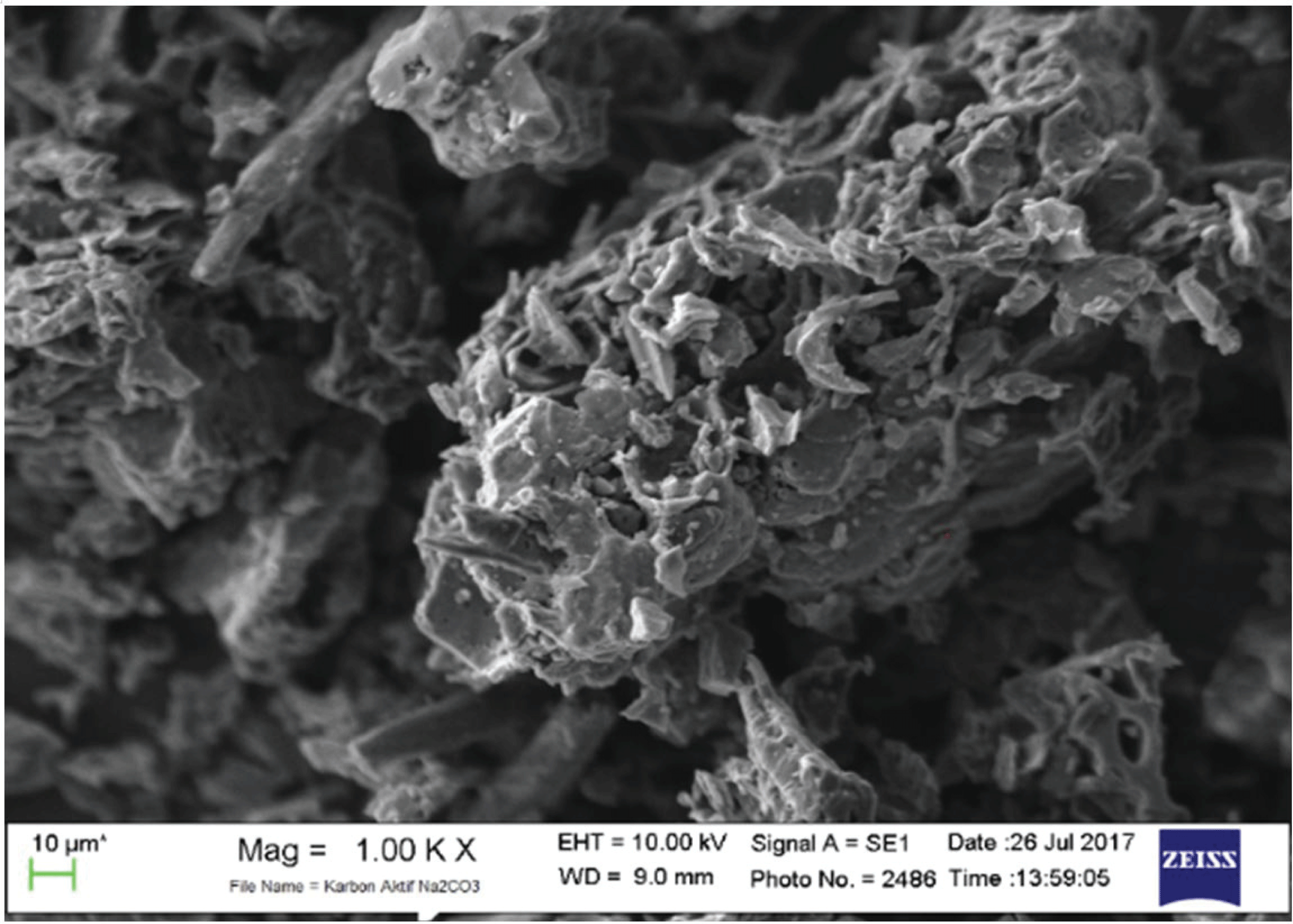
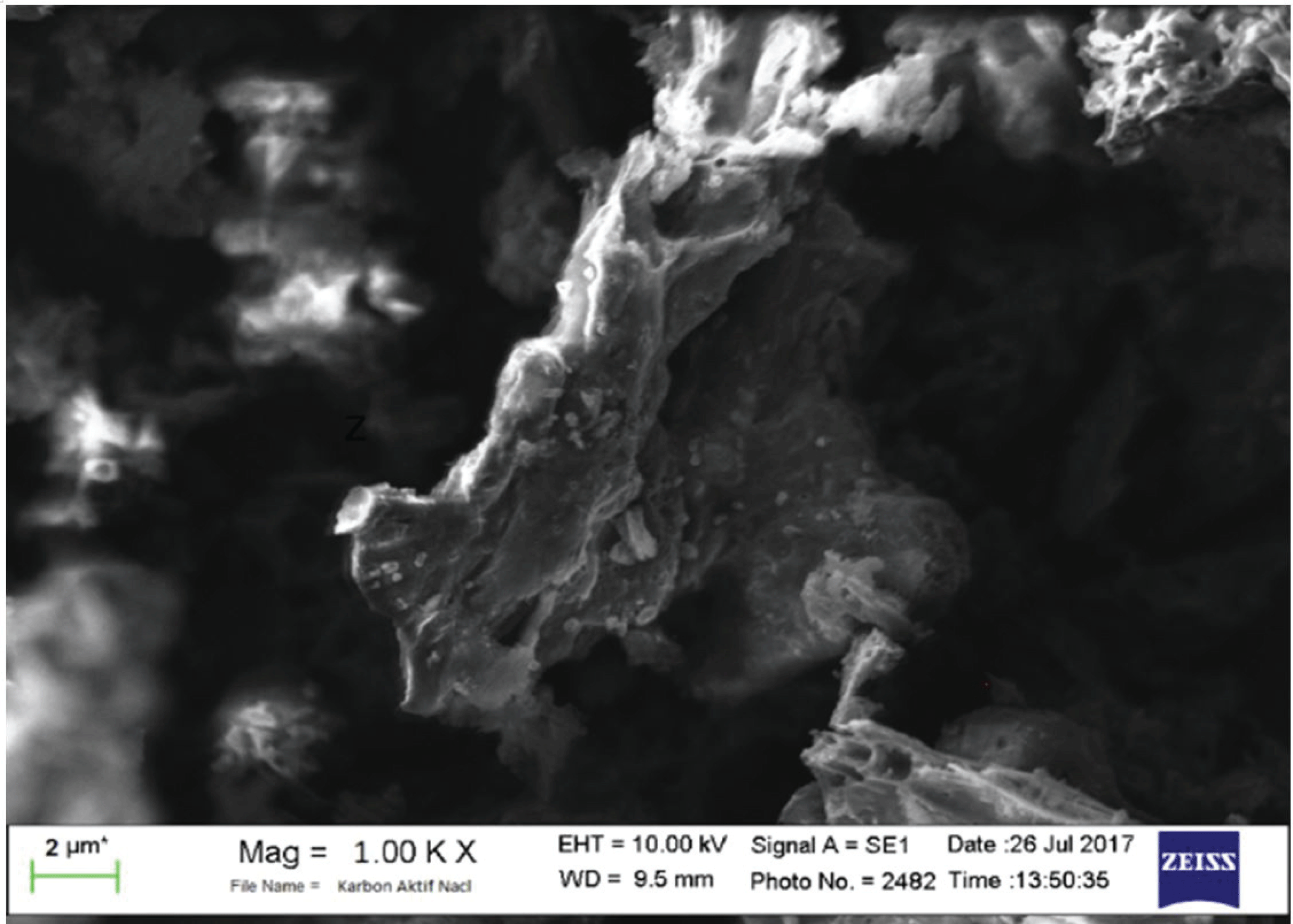
During carbonization, Na2CO3 decomposes from a metal compound of sodium to form carbon. The sodium metal atoms formed during activation and carbonization can enter carbon structures; this condition can expand the pores and create new porosity. Na2CO3 can be decomposed with carbon at above 400 °C. Thus, carbon is replace as CO and increases the surface area and pore volume. At high temperatures, pores form, and the mesoporous volume increases, but the absorption capacity of iodine decreases (Jin et al., 2012).
As shown in Figs. 8 and 9, more pores formed with greater pore cavity depth after using Na2CO3- activated carbons compared with NaCl-activated carbons. In addition, the Na2CO3 activator showed better iodine absorption (888.51 mg/g) compared with the NaCl activator (736.19 mg/g).
This study used the absorption method for spectroscopic observation; this method is based on differences in the absorption of infrared radiation. Fig. 10 present the results of FTIR analysis on the resulting activated carbon.
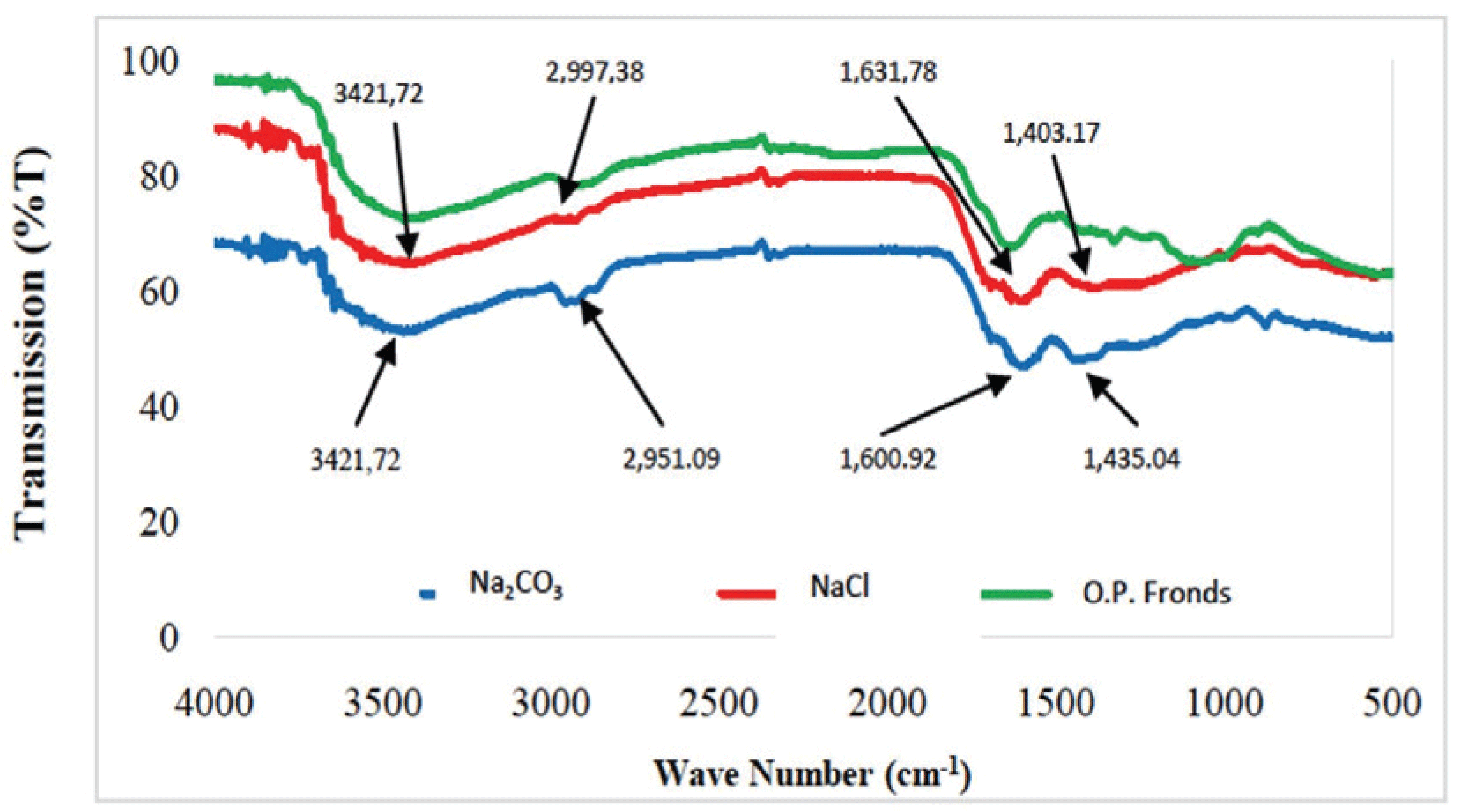
The absorption peak occurred at 3421.72 cm-1, which corresponds to by oil palm fronds and Na2CO3- and NaCl-activated carbons. The absorption peaks at 3500–3200 cm-1 (indicating to O-H spread) indicate the existence of hydroxyl (O-H) functional group. In the FTIR wave spectrum of activated carbon with Na2CO3 and NaCl activators, an absorption peak appeared with wave numbers of 1631.78 and 1600.92 cm-1, respectively. The absorption peaks occurring in the range of 1820–1600 cm-1 indicate the presence of C=O groups, which are typical groups of activated carbon. The peaks also showed that oil palm fronds originated form carbon-active substances (Kamariya et al., 2016).
Activation of carbon using Na2CO3 and NaCl also formed a C=C bond. This result was proven by the observation of spectra at wave numbers of 1,435.04 and 1,403.17 cm-1. The absorption peaks at 1500–1400 cm-1 referring the presence of the C=C group. Absorption peaks with wave numbers of 2951.09 and 299.73 cm-1 indicate the presence of C-H groups (3000–2850 cm-1) and alkane compounds, respectively (Mitome et al., 2013). Four types of functional groups, namely, C=O, C=C, C-C, and C-H groups, are contained in palm oil activated carbonyl.
4. CONCLUSION
The depth of the pore cavity is greater by using sodium carbonate activator than sodium chloride. This is also proved by Iodine number using sodium carbonate activator higher than sodium chloride activator in the activated carbon produced at temperature of 400 °C. Na2CO3 activator showed better iodine absorption (888.51 mg/g) compared to NaCl activator (736.19 mg/g). Na2CO3 has fulfilled the requirement of SNI 06-3730-1995.