1. INTRODUCTION
Licorice, the root and rhizome of Glycyrrhiza sp., has been traditional used as a medicinal herb and a natural sweetener. It prevents gastrointestinal ulcers (Momeni et al., 2014; Jalilzadeh-Amin et al., 2015); neutralizes toxic properties of compounds (Isbrucker and Burdock, 2006; Gong et al., 2015); and has antiviral (Wang et al., 2013; Fukuchi et al., 2016), antibiotic (He et al., 2006; Gupta et al., 2008; Lau and Plotkin, 2013; Chakotiya et al., 2016; Rohinishree and Negi, 2016), antifungal (Messier and Grenier, 2011; Seleem et al., 2016; Sharma et al., 2016), and anticancer activities (Shen et al., 2015; Fukuchi et al., 2016). Over the past 20 years, the number of studies on the use of licorice for oral health has steadily increased. Compounds in licorice improve bad breath by reducing the bacterial production of volatile sulfur compounds (Shin-ichi et al., 2012). Licorice has also been shown to ameliorate mouth dryness more effectively than water (Yu et al., 2016). In particular, licorice is thought to enhance oral health by inhibiting the growth of Streptococcus mutans, a primary causative agent of tooth decay (Segal et al., 1985; Hwang et al., 2004; Jain et al., 2013; Ajagannanavar et al., 2014). Consequently, the licorice lollipop was developed to control dental caries (Hum et al., 2011; Almaz et al., 2017).
Oral bacteria produce biofilms, called plaque, in the oral cavity. The oral cavity is a continuous flow system of food, drink, and saliva, and therefore, oral bacteria would be easily washed away without the ability to form a biofilm. Nonetheless, the oral cavity is a favorable environment for bacterial growth, and its many surfaces are coated with a plethora of bacteria, with >500–700 bacterial strains residing in the oral cavity (Rosan and Lamont, 2000; Dewhirst et al., 2010; Huang et al., 2011). Some of these bacteria are implicated in oral diseases. Moreover, specific oral bacteria strains have been associated with several systemic diseases. S. mutans, a gram-positive bacterium, is a regular member of the mature dental biofilm community. S. mutans has four serotypes c, e, f, and k based on its polysaccharides. Almost 70-80% of S. mutans found in the oral cavity is serotype c. However, under certain conditions, S. mutans can become prevalent and cause dental caries (Loesche, 1986; Ahn et al., 2008).
S. mutans produces insoluble glucan using sugars present in food. The insoluble glucan aids in plaque formation on the tooth surface where S. mutans exists (Krzysciak et al., 2014; Ren et al., 2016). S. mutans in the plaque cannot be easily removed by physical and chemical treatments (Welin-Neilands and Svensater, 2007; Bowen and Koo, 2011). The plaque also facilitates the intraoral adhesion of other bacteria, and these bacteria have been reported to cause not only oral diseases but also other complications, including endocarditis (Berbari et al., 1997), pneumonia (Scannapieco, 1999), low birth weight (Buduneli et al., 2005), and cardiovascular disease (Beck et al., 1996). These previous studies have suggested that chemical inhibition of biofilm formation in the oral cavity may play an important biological role in human health. One way to inhibit biofilm formation is by killing bacterial cells (Kondo et al., 2007). Recently, plant extracts that inhibited biofilm formation without the bactericidal effect of S. mutans have also been reported (Ham and Kim, 2018).
Although many studies have reported the beneficial effects of licorice extracts on oral health, no systematic study has been conducted on the extraction methods for preparing licorice extracts for inhibiting the biofilm formation of S. mutans. In this study, the activity of licorice extract to inhibit the biofilm formation of S. mutans was investigated according to ethanol concentration of extraction solvent. In addition, the extraction of the chemicals inhibiting the biofilm formation according to the ethanol concentration was observed to explain the specific extraction conditions.
2. MATERIALS and METHODS
Streptococcus mutans serotype c was obtained from LG Household & Health Care Ltd (Daejeon, Korea). Brain heart infusion (BHI) broth (BD Biosciences Korea, Seoul, Korea) was used for growing S. mutans. BHI agar was prepared by adding 1.5% agar to BHI broth. BHI-S medium, the biofilm formation medium, was prepared by supplementing BHI broth with 10-g/L sucrose. Glycyrrhizin (product number: G0150), glycyrrhetic acid (product number: G0149), and glucuronic acid (product number: G0302) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Liquiritin was purchased from Kono Chemical Co., Ltd. (Xian, China). Glycyrrhizin was dissolved in 50% ethanol, liquiritin and glycyrrhetic acid were dissolved in dimethyl sulfoxide, and glucuronic acid was dissolved in distilled water before use, respectively.
Licorice was purchased from Jiundang Oriental Medicine Co. (Seoul, Korea). The dried licorice extracts were prepared using licorice pulverized to a diameter of ≤1 mm. Subsequently, 10 g of the powdered licorice were mixed with 50-mL of water and ethanol and incubated at 50°C for 3 h. The content of ethanol in the extract solution was indicated in each experiment. The residual solid materials were removed by filtration (Whatman filter paper Grade 1). The filtrates were concentrated using a rotary evaporator (RV10, IKA® Korea, Ltd., Seoul, Korea). Without making powder, the original volume of the pre-concentrate extract was restored by adding distilled water. Insoluble materials were removed by centrifugation at 16,810 × g for 20 min and filtration through a syringe filter (25-mm diameter, cellulose acetate, 0.20-μm pore size; GVS North America, Inc., Sanford, ME, USA). The transparent extracts were stored at −80°C.
S. mutans, which was stored at −80°C, was streaked onto a BHI agar plate and cultured at 37°C for 2 days. A single colony was inoculated into 5-mL BHI broth and incubated at 37°C for 24 h without shaking. The cell density of cultured S. mutans was measured at an absorbance of 600 nm (Abs600) using an Optizen 2120 UV Plus spectrophotometer (Mecasys Co., Ltd., Daejeon, Korea). A 96-well polyvinyl chloride microplate containing 100-μL BHI-S medium was inoculated with the cultured cells at an optical density of 0.05 at Abs600. After incubation at 37°C for 24 h, the cell density was measured at Abs595 using an Opsys MR microplate reader (Dynex Technologies, Chantilly, VA, USA). After removing planktonic cells with distilled water, 100 μL of 1% crystal violet solution was dispensed into each well and allowed to stand for 15 min. After washing gently three times with distilled water, 100 μL of 95% ethanol was added to each well. After incubating for 15 min to allow the elution of the stained crystal violet, Abs595 was measured using an Opsys MR microplate reader. The one-way analysis of variance was used to distinguish differences from controls at the 95% confidence level by Tukey test using SPSS software (Ver. 23.0, SPSS Inc., Chicago, Illinois, USA).
To observe biofilm formation in a flow state similar to the oral cavity, biofilm formation of S. mutans was monitored in a continuous flow cell system. The flow cell system was purchased from the Center for Biomedical Microbiology (Technical University of Denmark, Lyngby, Denmark). The flow cell channel was covered with a polyvinyl chloride coverslip on which a biofilm was formed. Licorice extracted with 50% ethanol was added to the BHI-S medium at the indicated concentration.
As a preculture, S. mutans was inoculated into 5-mL BHI broth and incubated at 37°C for 24 h without shaking. The precultured cells were diluted to an optical density of 0.05 at Abs600, and 350 μL of diluted cells were inoculated into each channel. The coverslip was placed facing downward for 1 h, giving the cells a chance to attach to the surface of the coverslip. The flow cell system was turned on with the coverslip pointing upward and cultured for 24 h at a constant flow rate of 13 mL/h. The biofilm medium was continuously supplied to the flow cell system using a peristaltic pump (Masterflex L/S Digital Drive EW- 07523-90, Masterflex L/S 12-channel, 8-roller cartridge pump head EW-07519-25, Masterflex L/S small cartridges EW-07519-85, Cole-Parmer Instrument Company, LLC., Vernon Hills, IL USA). The biofilm formed under the coverslip was observed using an Axio Scope.A1 microscope (Carl Zeiss Co., Ltd., Seoul, Korea) equipped with ZEN microscope software (Carl Zeiss Co., Ltd).
For analysis of glycyrrhizin and glycyrrhetic acid in licorice extracts, the method of a previous study (Wang and Yang, 2007) has been used. The equipment of high performance liquid chromatograph was Acme 9000 (YL Instruments Co., Ltd, Anyang, Korea). The analytical column used was YMC-Triart C18 (Product code: TA12S05-2546WT, YMC Korea Co., Ltd, Seongnam, Korea). The flow rate of mobile phase was 1.2 mL/min. The wavelength of light for UV detector was 254 nm. The column temperature was 40°C. The injection volume of sample was 10 μL. The licorice extract was separated using a gradient mobile phase according to a previous study (Wang and Yang, 2007). The data were analyzed with Autochro-3000 software (version 2.0.0).
3. RESULTS and DISCUSSION
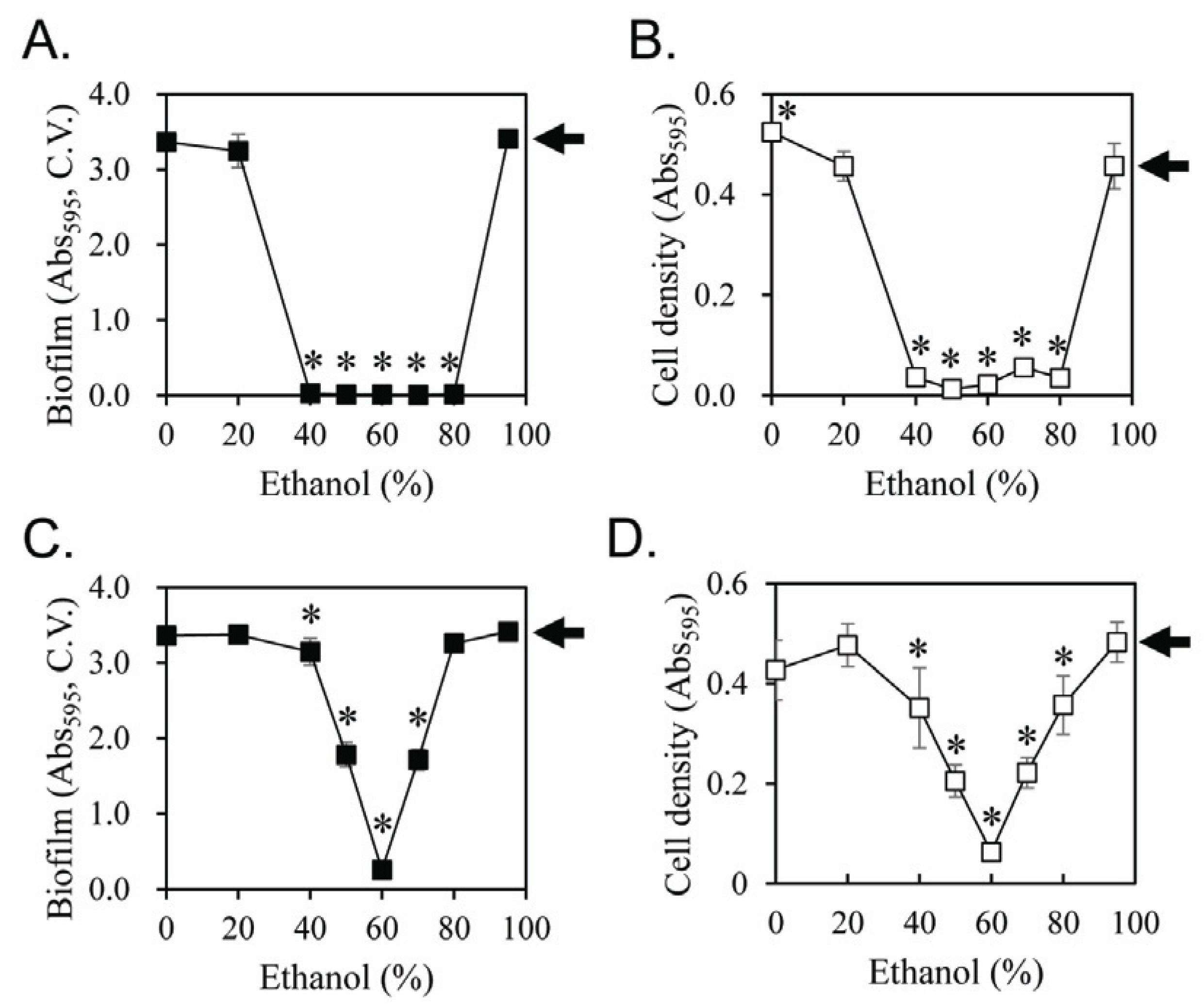
Licorice has recently been shown to be effective against dental caries and oro-dental diseases (Touyz, 2009; Messier et al., 2012). In previous studies, various solvents were used for licorice extraction (He et al., 2006; Al-Turki et al., 2008; Nitalikar et al., 2010; Ahn et al., 2012; Rodino et al., 2015; Karahan et al., 2016). In the present study, water and ethanol were tested to prepare the licorice extract for evaluating its inhibitory activity on the biofilm formation of S. mutans. Biofilm formation and cell growth inhibition were measured as a function of the ethanol content in the extracting solvent (Fig. 1). When 5% by volume of the extract was treated with the culture, a robust inhibitory effect on biofilm formation was observed using licorice extracts made with 40%–80% ethanol (Fig. 1A). The cell growth of S. mutans was correlated with biofilm formation (Fig. 1B). To determine the range of effective ethanol concentrations more accurately, we tested with extracts having 1.25% of the cell culture volume tested (Fig. 1C and 1D). Clearly, the most effective inhibitory activity on the biofilm formation of S. mutans was shown the licorice extract made with 60% ethanol.
To confirm the effect of ethanol content in the extracting solvent in Fig. 1, the amount of the solvent required for inhibiting the biofilm formation of S. mutans was examined (Fig. 2). The licorice extracts prepared using water or 95% ethanol as the extraction solvent showed no inhibitory effect on biofilm formation, irrespective of the amount of solvent (Fig. 2A and 2E, respectively). In case of 50% ethanol, the inhibitory activity on biofilm formation started appearing using the licorice extract prepared with 100-mL solvent and most biofilm formation was inhibited by licorice extracts prepared with a solvent volume of less than 50-mL (Fig. 2C). The effect of the licorice extract on the cell growth of S. mutans was comparable to that on biofilm formation (Fig. 2D and 2F), except for the water extract (Fig. 2B). The results in Fig. 2 confirmed that licorice extracted with around 50% aqueous ethanol effectively inhibited the biofilm formation of S. mutans in Fig. 1. The yield in extraction was measured according to the ethanol content (Table 1). There was no significant change in the solid yield up to 70% ethanol, but it decreased rapidly at more than 80% ethanol. This result support that there was a large portion of water soluble materials in licorice (Kondo et al., 2007). However, the specific inhibitory effect on the biofilm formation shown in the licorice extract using 60% ethanol as the extraction solvent cannot be explained by the yield of the extract.
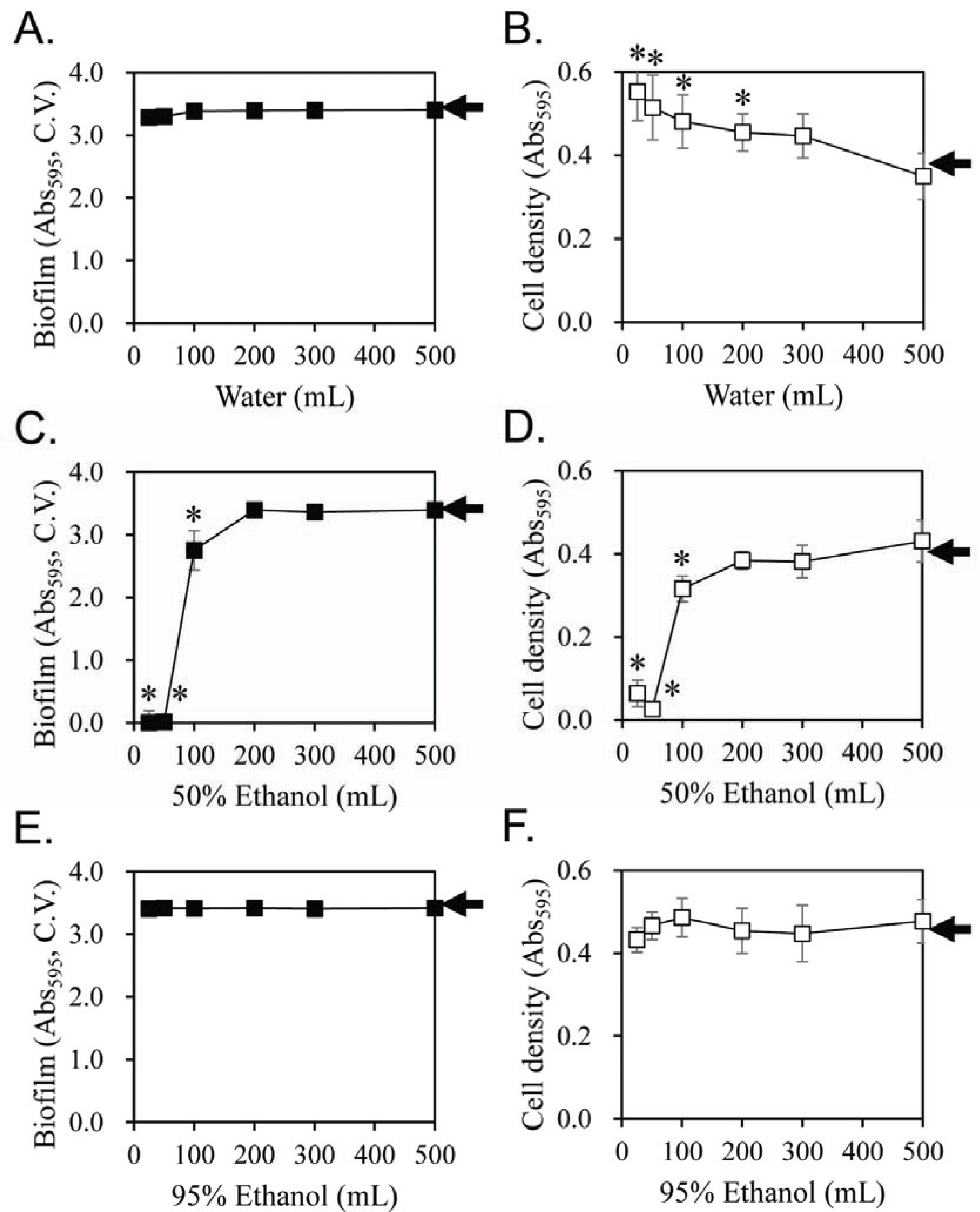
The inhibitory effect on biofilm formation was not observed using the water extract, which is the traditional method for making licorice extract, or the 95% ethanol extract. The biofilm formation of S. mutans was inhibited by extracts prepared with 60% ethanol content. Considering that ethanol has polarity like water, this ethanol concentration-specific extraction condition is highly unusual.
The inhibitory activity of licorice on the biofilm formation of S. mutans was evaluated in a microplate assay and in a flow cell system using the 50% aqueous ethanol licorice extract (Fig. 3 and Fig. 4, respectively). Biofilm formation was inhibited by 0.5-g/L licorice extract, and most of the biofilm formation was inhibited at concentrations around 1.5 g/L (Fig. 3A). Cell growth was correlated with the biofilm formation (Fig. 3B), as observed in Fig. 1.

The inhibitory effect of licorice extract on the biofilm formation of S. mutans was confirmed using a continuous flow cell system (Fig. 4). Consistent with the microplate experiments, the inhibitory activity on the biofilm formation began to appear at 0.5-g/L licorice extract.
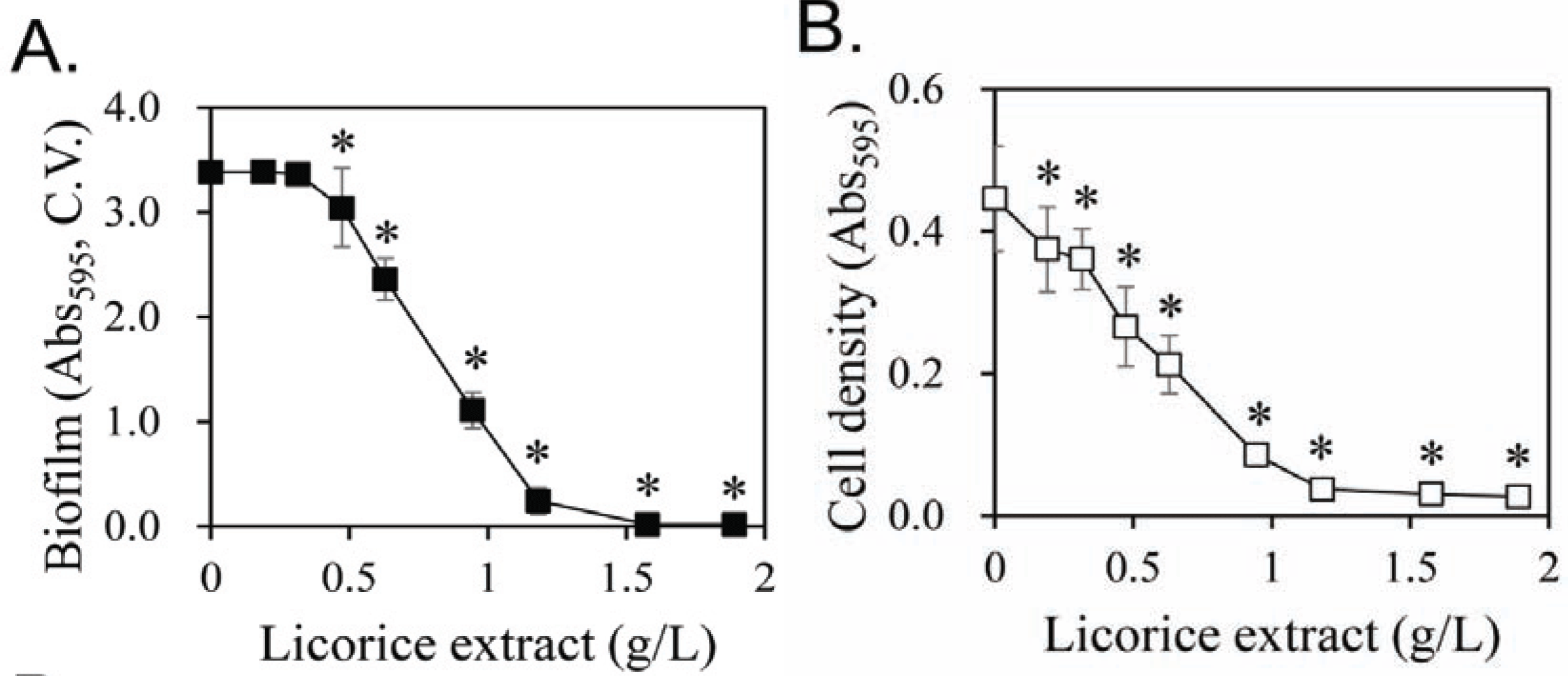
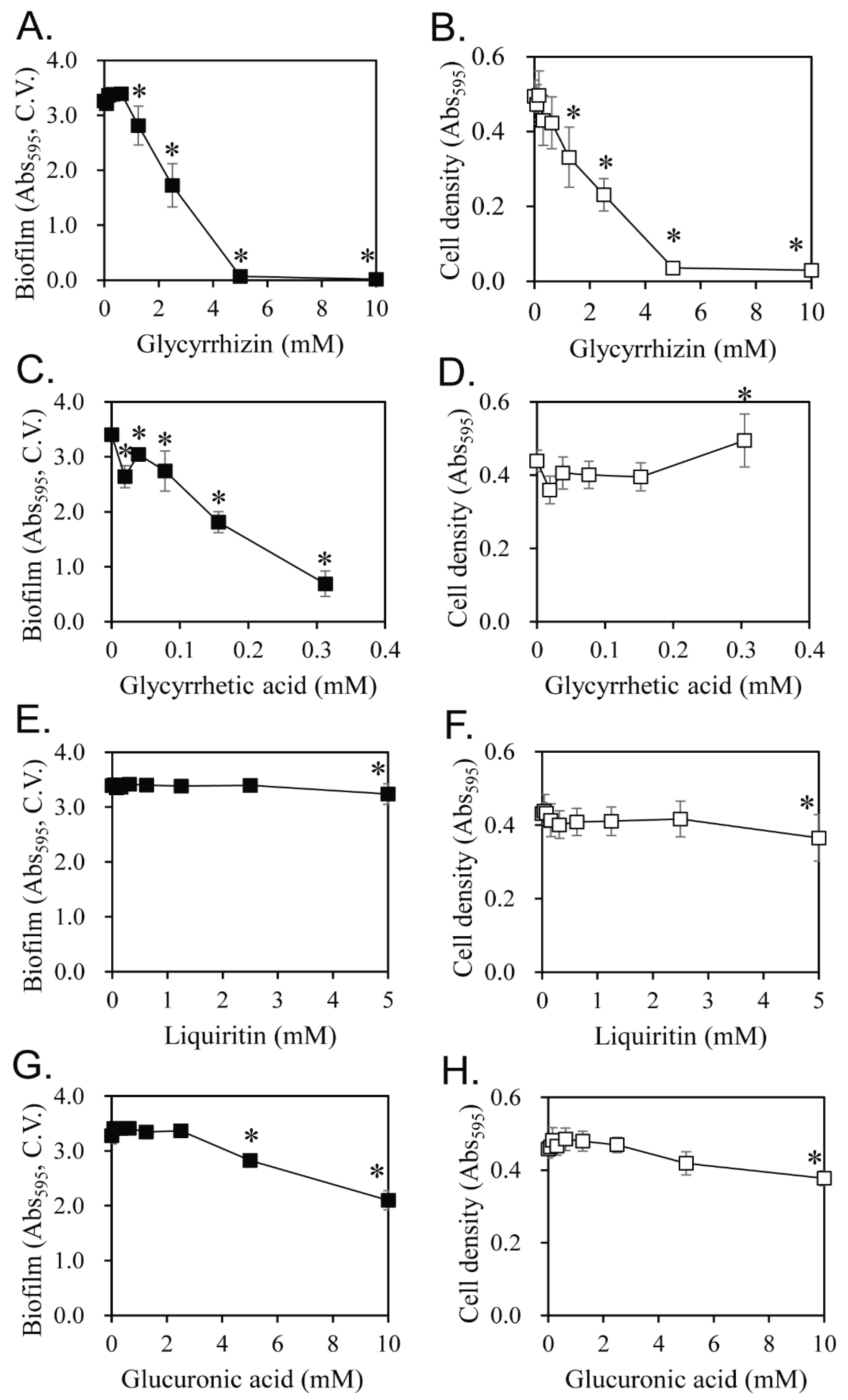
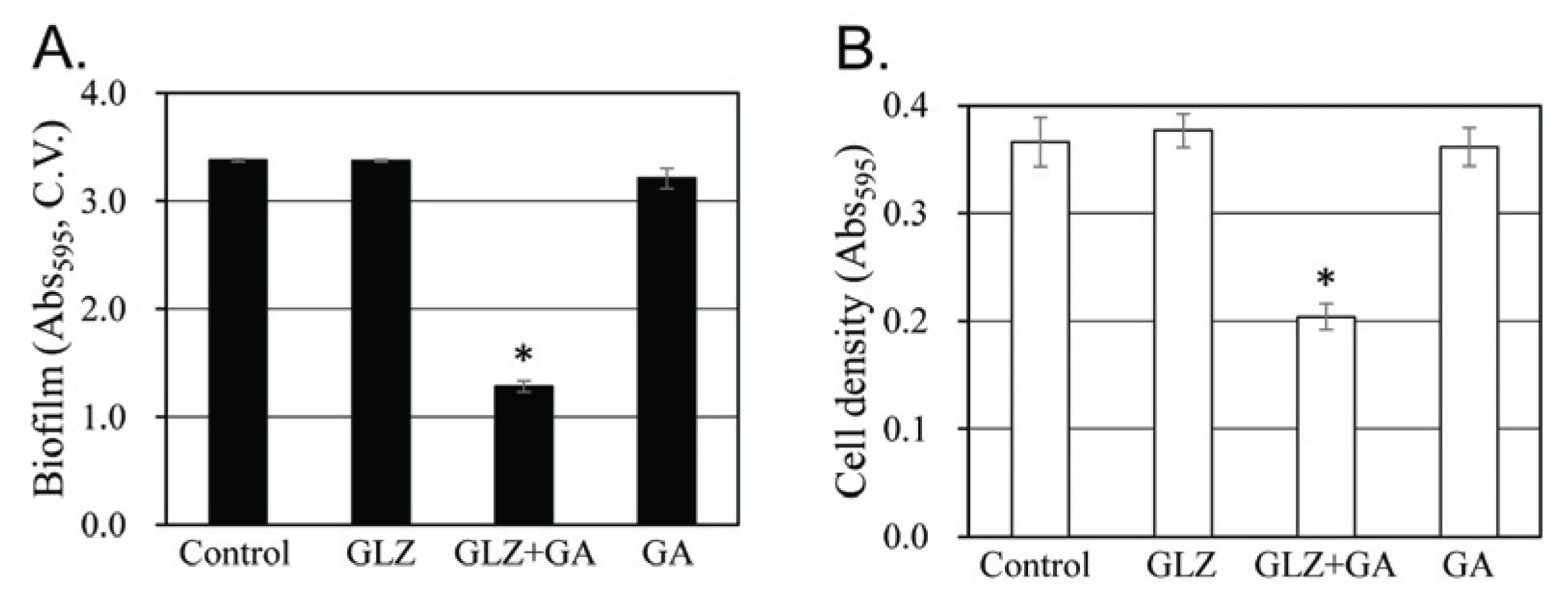
Previous studies on the treatment of oral diseases using licorice have suggested that licorice may kill S. mutans cells directly (Ajagannanavar et al., 2014; Wang et al., 2015) and/or inhibit plaque formation (Segal et al., 1985; Ahn et al., 2012). Several components of licorice have been reported to exhibit physiological functions that kill oral microorganisms (He et al., 2006; Kondo et al., 2007). We also evaluated the inhibitory activity of the main components of licorice on biofilm formation (Fig. 5). Glycyrrhizin and glycyrrhetic acid showed strong inhibitory activity, but liquiritin and glucuronic acid had no and weak inhibitory activity, respectively. Therefore, the content of glycyrrhizin and glycyrrhetic acid in extracts were analyzed according to the ethanol concentration of the extract solvent (Table 1). Glycyrrhizin is extracted at high concentration in water and 50% ethanol and at very low concentration in 95% ethanol. Therefore, glycyrrhizin, a major component of licorice, cannot explain the specific strong biofilm formation inhibition in 60% ethanol extracts. In the case of glycyrrhetic acid, it was extracted at 0.001 g/L by water. However, 50% ethanol extracts glycyrrhetic acid at 0.046 g/L, which is 46 times higher than water extract. Glycyrrhetic acid was not detected in the 95% ethanol extract. This can partially explain the specific strong biofilm formation inhibition in the 60% ethanol extract. In Fig. 5, the inhibitory activity of glycyrrhetic acid on biofilm formation is around ten times stronger than that of glycyrrhizin. However, the content of glycyrrhetic acid in the 50% ethanol extract is less than 1% of glycyrrhizin, and it is not enough amount to explain the specific strong biofilm formation inhibition in the 60% ethanol extract, despite 10-fold strong inhibitory activity on biofilm formation.
In order to explain the specific strong biofilm formation inhibition in the 60% ethanol extract, the synergistic inhibitory effect of glycyrrhizin and glycyrrhetic acid was evaluated (Fig. 6). When cells were treated with a mixture of 1.25 mM glycyrrhizin and 0.075 mM glycyrrhetic acid, which had no inhibitory activity individually, about 62% of biofilm formation was inhibited. When glycyrrhizin or glycyrrhetic acid was treated at concentrations of 2.5 mM or 0.15 mM, respectively, which were twice the treatment concentration, both chemicals showed around 47% inhibition of biofilm formation (Fig. 5). The inhibitory activity of the two compound mixture, 62%, shows a synergistic effect, compared with 47%, which is the inhibitory activity of treating each compound with a double concentration. Considering both a strong inhibitory activity of glycyrrhetic acid and its synergistic effect with glycyrrhizin on the biofilm formation of S. mutans, ethanol concentration specific extraction for glycyrrhetic acid may be a major reason for the strong inhibitory activity of 60% ethanol extracts of licorice.
Inhibition of biofilm formation mediated by licorice could be attributed to the prevention of bacterial growth. Previous studies have shown that glycyrrhizin from licorice inhibits biofilm formation of S. mutans by inhibiting or killing the cell growth (He et al., 2006; Kondo et al., 2007). However, in this study, licorice extract with 60% ethanol is more effective when considering only inhibition of biofilm formation. This ethanol concentration specific extracts can be explained by the specific ethanol concentration extraction for glycyrrhetic acid from licorice and its synergetic inhibitory effect with glycyrrhizin. In order to maximize the effect of inhibiting the formation of plaque, a biofilm of S. mutans causing tooth decay, the extraction conditions of licorice were suggested and the corresponding chemical, glycyrrhetic acid, was presented in this study. In addition to the antioxidant activities (Nam et al., 2018; Kim et al., 2017; Kim et al., 2018), inhibition of microbial biofilm formation suggests new functionality of plant extracts.
4. CONCLUSION
Licorice is a traditional medicine with a sweet taste. Interestingly, it has been known that licorice is effective in preventing tooth decay. This study showed that licorice extract inhibits the cell growth and biofilm formation of S. mutans, which is a major cause of tooth decay. We found that licorice extracts prepared using around 60% ethanol effectively inhibited the biofilm formation of S. mutans, whereas those prepared using general extraction methods with water showed no effect on the biofilm formation. The ethanol concentration specific inhibitory ability of licorice extracts was due to the strong inhibitory activity of glycyrrhetic acid and its synergistic inhibitory activity with glycyrrhizin on the biofilm formation of S. mutans.








