1. INTRODUCTION
Streptococcus mutans causes dental caries by attaching to gum and teeth surfaces and producing biofilm (plaque) and acid (Loesche, 1986; Ahn et al., 2008). The biofilm produced by S. mutans helps the bacterium and other microbes to attach oral surfaces, leading to diseases such as gingivitis and periodontitis. Therefore, S. mutans flourishing in the oral cavity may result in complications from additional infectious microorganisms (Ahn et al., 2008); these include endocarditis (Berbari et al., 1997), pneumonia (Scannapieco, 1999), systemic diseases such as cardiovascular disease (Beck et al., 1996; Li et al., 2000), and low birth weight and preterm birth rate (Buduneli et al., 2005). The microorganisms attached to biofilms are more resistant to physical, chemical, and biological treatments than the planktonic cells (Welin-Neilands and Svensater, 2007; Bowen and Koo, 2011). Therefore, it is important to inhibit biofilm formation to prevent various diseases in which S. mutans cannot be eliminated.
For removing biofilm in the oral cavity, physical methods (Loe, 2000), such as tooth brushing and flossing, and chemical methods (Wolff, 1985; Glassman et al., 2003; Paula et al., 2010), such as chlorhexidine-based oral cleansers, are used. Because physical methods often leave residual microorganisms, it is desirable to use chemical methods additionally to efficiently eliminate biofilms. Most chemicals used in chemical hygiene modalities have high antimicrobial activity and sometimes exhibit side effects such as tooth coloring, tartar formation, and oral mucosal dissolution (Wolff, 1985; Eley, 1999). Continuous usage of chemical agents with bactericidal properties also alters the microbial flora in the oral cavity (Walker, 1996; Goncalves et al., 2007).
Recently, there has been growing interest in natural compounds that can inhibit bacterial growth or biofilm formation without such side effects (Dixon, 2001; Simoes et al., 2009; Saleem et al., 2010). Plants produce diverse secondary metabolites that protect them from herbivores, insects, microbes, and other plants (Harborne, 1990; Dixon, 2001) and possess additional biofunctions (Jeon et al., 2011; Palombo, 2011; Jeong et al., 2017; Jung et al., 2017; Nam et al., 2018). However, the extracted secondary metabolites depend on various extraction factors, such as time, temperature, and solvent, which eventually affect the efficacy of the resultant extracts (Liyana-Pathirana and Shahidi, 2005; Kim et al., 2017; Kim et al., 2018).
In this study, we screened plant extracts that specifically inhibited the biofilm formation of S. mutans without antibiotic effects.
2. MATERIALS and METHODS
Dried selected plants were purchased from an herbal medicine shop (Jiundang, Seoul, Korea). These were ground to a size of ≤1 mm for extraction. The resultant plants powders (30 g) were mixed with methanol (300 ml) and incubated at 50°C for 3 h. After incubation, the residual plant powders were removed via filtration using Whatman® qualitative filter papers (Grade 1, GE Healthcare Life Science, Seoul, Korea). Filtered extracts were concentrated using a rotary evaporator (RV10, IKA® Korea, Ltd., Seoul, Korea). The semi-solid extracts were then dried for at 60°C for 1 week. Subsequently, the dried extracts were stored in a freezer at −80°C.
S. mutans was generously provided by LG Household & Health Care Ltd. (Daejeon, Korea) and was stored at −80°C in 25% glycerol. Bacto™ brain heart infusion (BHI) broth was purchased from BD Biosciences Korea Ltd. (Seoul, Korea). BHI agar medium was prepared by adding 15 g/l agar to BHI broth. BHI-S medium was prepared by supplementing BHI broth with 1% sucrose (Song et al., 2007). S. mutans was streaked onto BHI agar plate and cultured at 37°C for 2 days. A single colony was inoculated in 5 ml BHI broth and incubated at 37°C for 1 day. The cell density was measured using Optizen 2120 UV plus spectrophotometer (Mecasys Co., Ltd., Daejeon, Korea) as the absorbance at 600 nm.
Dried extracts were dissolved in methanol in a concentration of 50 g/l, followed by centrifugation at 13,000 rpm for 20 min. The resultant supernatants were filtered using a syringe filter (cellulose acetate; 25 mm diameter, 0.2 μm pore size; GVS Korea Ltd., Namyangju, Korea). The resultant extract library was stored at −80°C until use.
The selected plants from the first screening were ground to a size of ≤1 mm. The powders of nine selected plants (10 g each) were mixed with 100 ml of water, 50% ethanol, or 95% ethanol and incubated at 50°C for 3 h. The residual powders after incubation were removed via filtration using Whatman® qualitative filter papers. Each water extract was directly used for activity evaluation after filtration using a syringe filter (GVS Korea Ltd.) Additionally, 50% and 95% ethanol extracts were concentrated using RV10 rotary evaporator, following which water was added to the original volume. After centrifugation at 13,000 rpm for 20 min, the water-soluble component of each extract was finally separated by filtration using a syringe filter (GVS Korea Ltd.). All prepared extracts were stored at −80°C.
The biofilm amount was quantitatively measured as previously described (Ham and Kim, 2016). The extracts (0.5 g/l) were added to the wells of a 96-well polyvinyl chloride microplate containing 100 μl of BHI-S medium per well. S. mutans was inoculated in the wells at a cell density of 0.05, as measured at an absorbance at 600 nm. The microplates were incubated at 37°C for 24 h to permit biofilm formation. Cell density after 24 h was measured at 595 nm using Opsys MR™ microplate reader (Dynex Technologies Inc., Chantilly, VA, USA). Planktonic cells were rinsed with water, and 100 μl of 1% crystal violet was added to each well and incubated at room temperature for 15 min. The wells were then rinsed thrice with water, and 100 μl of 95% ethanol was added to each well. After allowing crystal violet to dissolve in the biofilm for 15 min, the absorbance was measured at 595 nm using Opsys MR™ microplate reader.
3. RESULTS and DISCUSSION
Various solvents, such as methanol, ethanol, acetone, and water, can be used to extract bioactive compounds from plants. Among these, methanol is used to extract a wide variety of compounds (Dai and Mumper, 2010). The inhibitory activity of methanol extracts on biofilm formation by S. mutans was evaluated (Fig. 1). Among the 140 extracts that were tested, extracts from nine plants, Melonis Pedicellus (sample number: 12 in Fig. 1), Agastachis Herba (sample number: 13 in Fig. 1), Mori Cortex Radicis (sample number: 70 in Fig. 1), Diospyros kaki leaves (sample number: 77 in Fig. 1), Agrimoniae Herba (sample number: 88 in Fig. 1), Polygoni Multiflori Radix (sample number: 108 in Fig. 1), Lycopi Herba (sample number: 117 in Fig. 1), Elsholtziae Herba (sample number: 129 in Fig. 1), and Schizonepetae Spica (sample number: 131 in Fig. 1), inhibited biofilm formation by S. mutans.
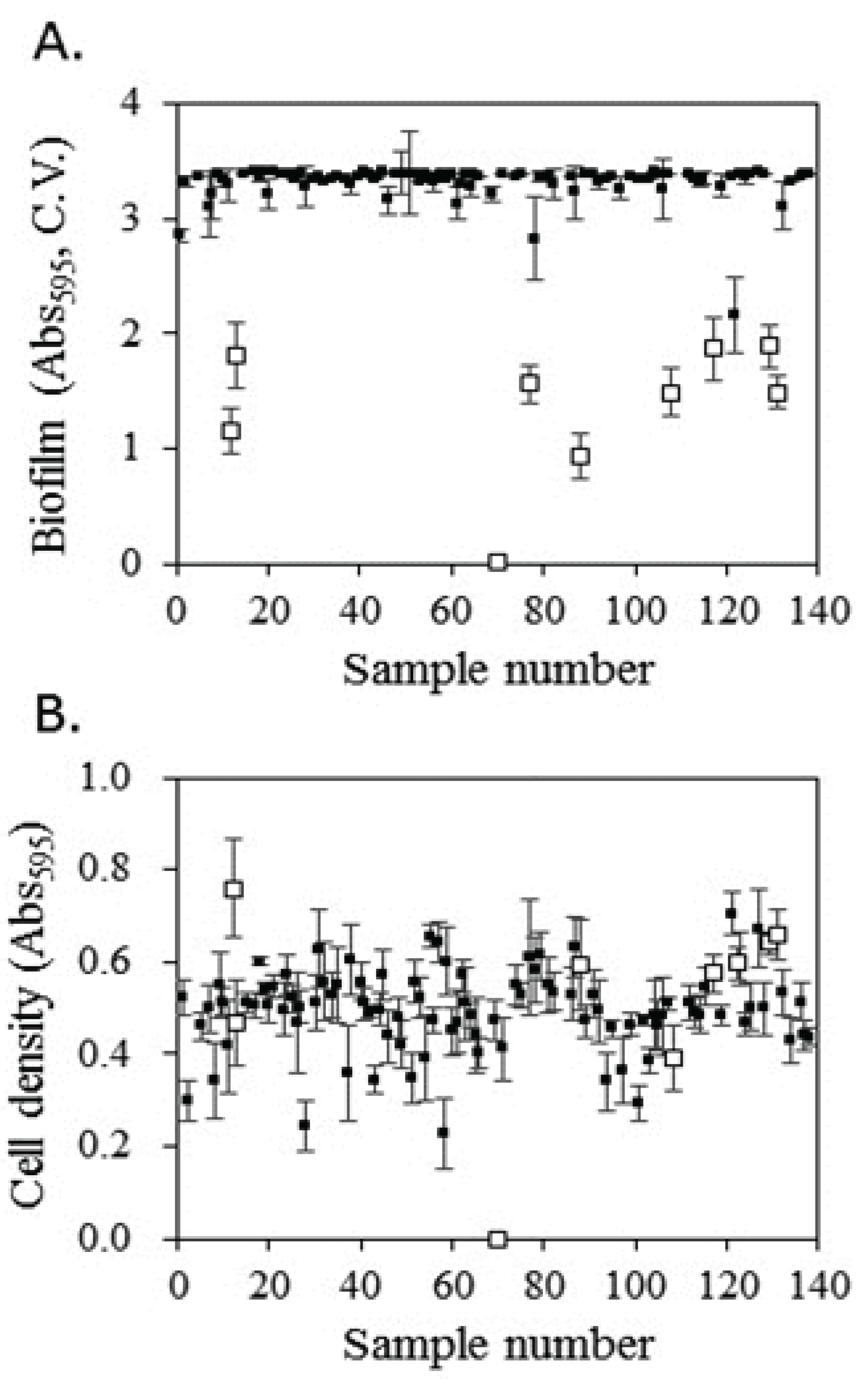
The selected extracts, excluding Mori Cortex Radicis extract, did not significantly affect the growth of S. mutans. Mori Cortex Radicis extract exhibited strong antibacterial activity; therefore, this extract appeared to reduce biofilm formation by significantly reducing the number of bacterial cells. None of the other extracts exhibited growth-inhibitory activity; however, biofilm formation was selectively inhibited despite sufficient bacterial growth. Among the extracts excluding Mori Cortex Radicis extract, the strongest inhibition of biofilm formation were exhibited by Agrimoniae Herba (72% inhibition) extract; the inhibitory activities of the remaining extracts on biofilm formation increased in the following order: Melonis Pedicellus, Polygoni Multiflori Radix, Schizonepetae Spica, Diospyros kaki leaves, Agastachis Herba, Lycopi Herba, and Elsholtziae Herba.
Inhibitory effects of nine selected methanol extracts on biofilm formation by S. mutans were measured via serial dilution (Fig. 2). The methanol extract of Mori Cortex Radicis, which exhibited both antimicrobial and biofilm formation inhibitory activities, displayed strong inhibitory activity at a low concentration of 0.0625 g/l. At a concentration of 0.03 g/l, the biofilm formation inhibitory activity was approximately 90% compared with approximately 24% at a concentration of 0.015 g/l. No inhibitory activity was observed at concentrations less than 0.0078 g/l.
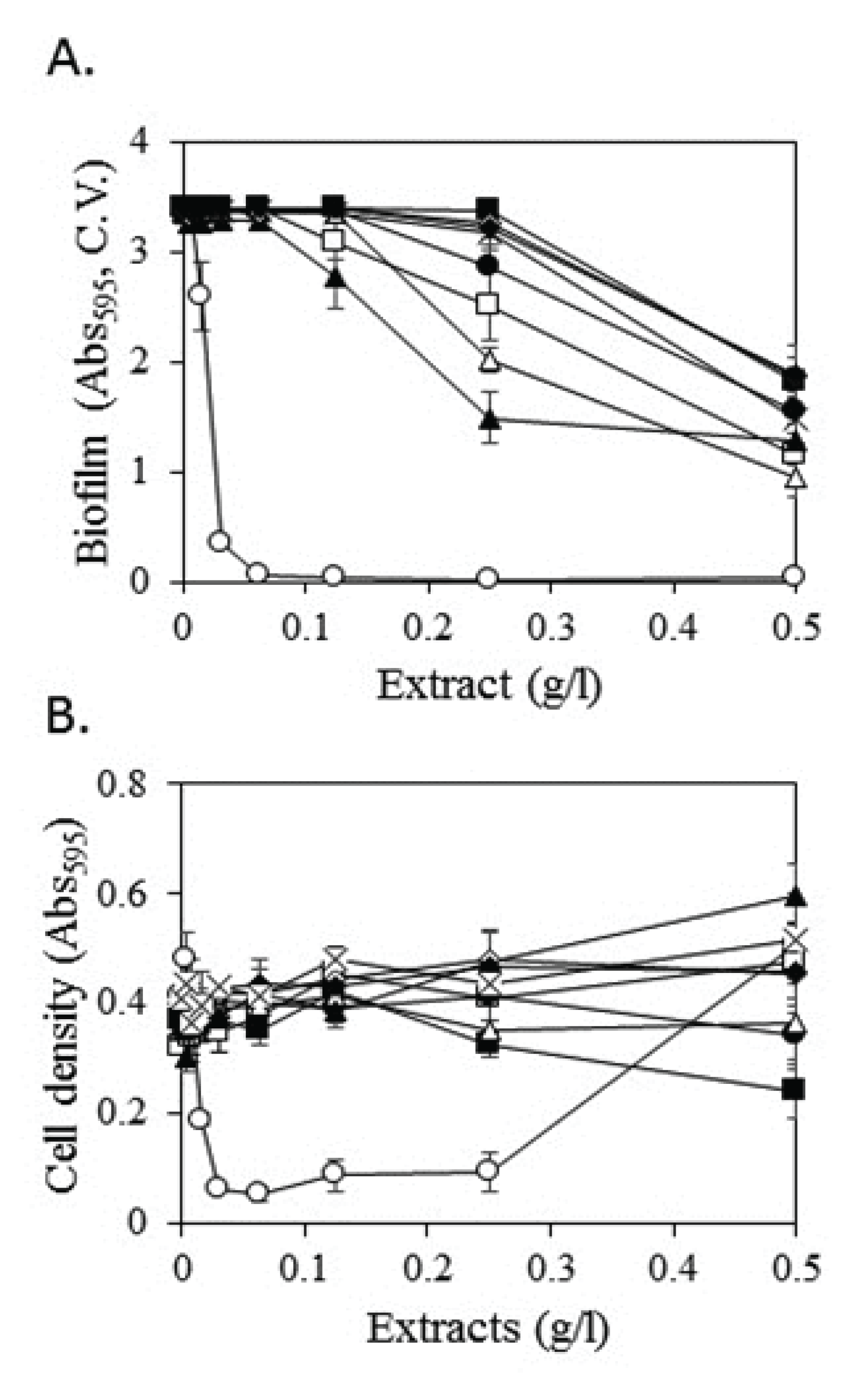
Methanol extracts of Agastachis Herba, Lycopi Herba, Elsholtziae Herba, and Schizonepetae Spica did not exhibit significant inhibitory activity against biofilm formation at 0.25 g/l, reducing biofilm formation by 56%, 40%, 26%, and 16%, respectively. At a concentration of 0.13 g/l, the extracts of Mori Cortex Radicis, Polygoni Multiflori Radix, and Melonis Pedicellus reduced biofilm formation by 99%, 13%, and 9%, respectively, whereas the remaining extracts displayed no inhibitory activity. At concentrations of less than 0.06 g/l, only Mori Cortex Radicis inhibited biofilm formation.
The nine selected plants were extracted with water and 50% and 95% ethanol. The inhibitory activity of water-soluble components isolated from the extracts was observed by redissolving these extracts in water (Table 1). Inhibitory effects on biofilm formation were observed for 50% ethanol extract of Diospyros kaki leaves (45%), water extract of Agrimoniae Herba (52%), 50% ethanol extract of Agrimoniae Herba (68%), water extract of Polygoni Multiflori Radix (29%), and 50% ethanol extract of Polygoni Multiflori Radix (56%). No inhibitory activity was observed for any of the 95% ethanol extracts.
The inhibitory activity of some extracts was further examined based on the concentration (Fig. 3). Regarding Mori Cortex Radicis, for which the methanol extract exhibited strong antibacterial activity and inhibitory activity against biofilm formation, water and 50% ethanol extracts displayed neither of the two activities (Fig. 3A). In addition to the methanol extract, the 50% ethanol extract of Diospyros kaki leaves inhibited biofilm formation (open circle in Fig. 3B). Specifically, at 0.425 g/l, the methanol extract of Diospyros kaki leaves inhibited biofilm formation by approximately 54%, whereas the 50% ethanol extract displayed no inhibitory activity. Methanol, water, and 50% ethanol extracts of Agrimoniae Herba and Polygoni Multiflori Radix inhibited biofilm formation (Fig. 3C and 3D, respectively). In particular, methanol and 50% ethanol extracts of Agrimoniae Herba displayed similar inhibitory effects, with both inhibiting biofilm formation by 55% at a concentration of 0.4 g/l. The water extract of Agrimoniae Herba suppressed biofilm formation by approximately 52% at 0.9 g/l, whereas no inhibitory activity was observed at 0.45 g/l. Concerning Polygoni Multiflori Radix, the methanol extract inhibited biofilm formation by 62% at 0.5 g/l and 56% at 0.25 g/l. These effects were stronger than those observed for 50% ethanol and water extracts, as the latter reduced biofilm formation only at a concentration of 0.9 g/l.
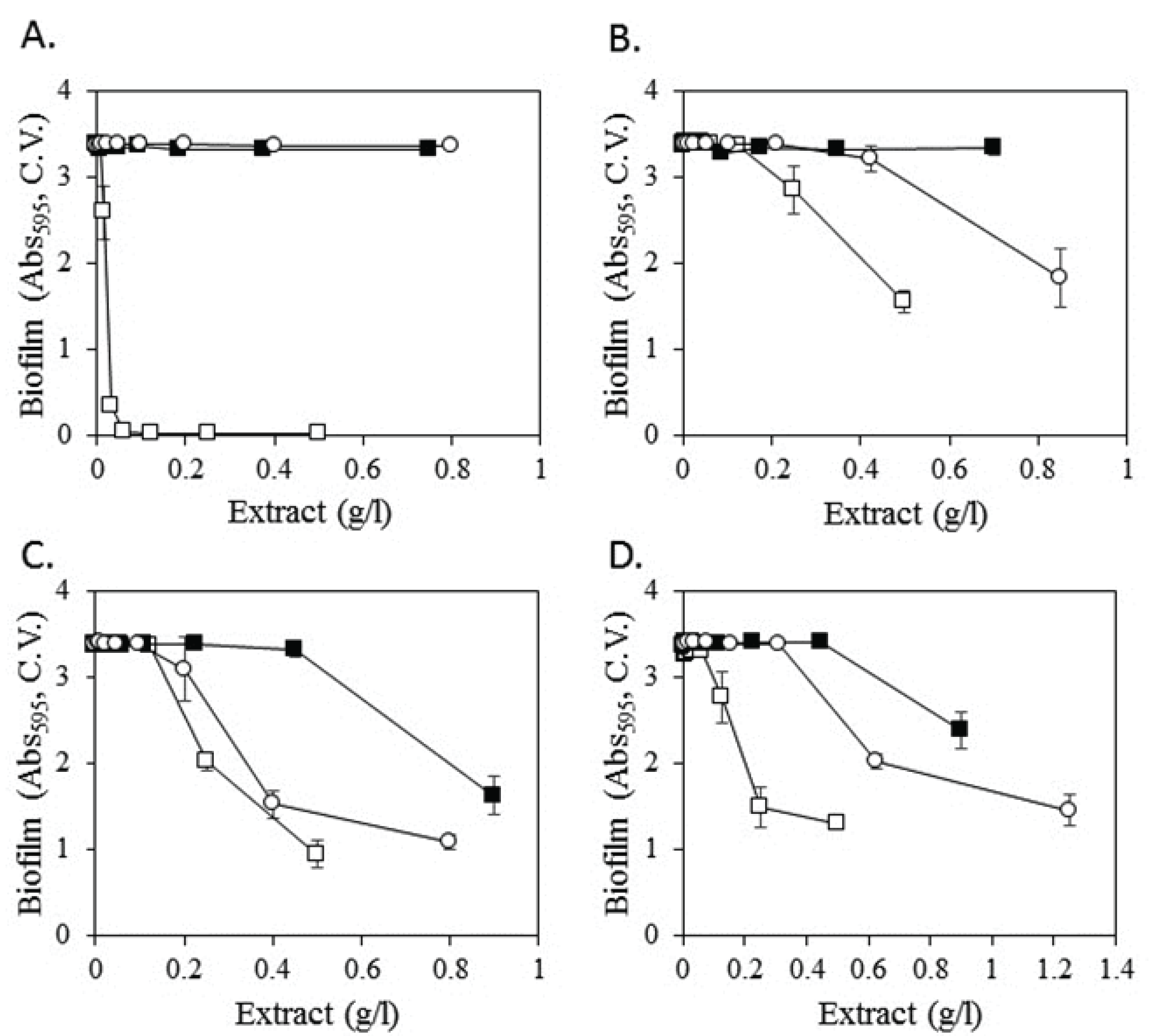
Among the 140 methanol extracts of plants, nine extracts listed in Table 1 inhibited biofilm formation by S. mutans. Biofilm formation may be suppressed by inhibiting cell growth, but only Mori Cortex Radicis exerted inhibitory effects on the growth of S. mutans. Some plant extract also showed antibiotic activity (Koo et al., 2002; Lee et al., 2014; Park et al., 2005). These observations suggest that the methanol extracts of the remnant eight plants contain biological chemicals that selectively inhibit biofilm formation by S. mutans without affecting cell growth.
Mori Cortex Radicis is obtained from the root bark of Morus alba L. The methanol extract of Mori Cortex Radicis displayed excellent inhibitory activities against biofilm formation, whereas water and ethanol extracts exhibited no such activities. These results suggest that cell growth and biofilm formation by S. mutans are strongly inhibited by compounds selectively extracted by methanol. Previous studies observed similar strong inhibitory effects of Mori Cortex Radicis against cell growth and biofilm formation by S. mutans; researchers suggested that the compounds exhibiting the these activities were sanggenon C (Park et al., 1990) and kuwanon G (Park et al., 2003). The results consistent with previous studies showed that the method of searching for inhibitory extracts in this study is working as intended.
The inhibitory effects of Diospyros kaki leaf extract on biofilm formation by Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium, Streptococcus mutans, and Streptococcus sanguinis have been described (Lokegaonkar and Nabar, 2010; M. Ramadan et al., 2017). It has been reported that 1-deoxynojirimycin, a compound present in Diospyros kaki leaves, possesses approximately 8-fold stronger antimicrobial and biofilm-inhibiting effects against S. mutans than the crude extract (Islam et al., 2008).
It has been reported that Diospyros kaki leaves inhibit the proliferation of cancer cells (Arakawa et al., 2014) and possess antiviral and antimicrobial activities (Jeong et al., 2009; Tomiyama et al., 2016). Kaempferol in Diospyros kaki leaves demonstrated antibacterial effects against S. mutans (Yamada et al., 1999). Agrimoniae Herba is the aerial part of Agrimonia pilosa Ledeb., and has been used as an anti-inflammatory (Taira et al., 2009; Jung et al., 2010), antioxidant (Zhu et al., 2009), and antibacterial agent (Yamaki et al., 1989). Polygoni Multiflori Radix is the tuberous root of Polygonum multiflorum Thunberg, a vine-like medicinal plant belonging to the Polygonaceae family. The leaves, tuberous roots, and rootstocks of this plant contain many biologically active compounds. P. multiflorum has been reported to have anticancer (Horikawa et al., 1994; Choi et al., 2007), antimicrobial (Zuo et al., 2008), anti-inflammatory (Dong and Jeon, 2009), anti-oxidative (Ip et al., 1997; Chan et al., 2003; Lv et al., 2007), and liver-protective properties (Guo et al., 2001; Huang et al., 2007). Tuberous roots of P. multiflorum (Polygoni Multiflori Radix) have been used in Oriental medicine. Its ethanol extract is known to inhibit the enzymatic activity of Ca2+-ATPase(Grech et al., 1994).
The 50% ethanol extract of Diospyros kaki leaves and water and 50% ethanol extracts of Agrimoniae Herba and Polygoni Multiflori Radix demonstrated inhibitory activity against biofilm formation. Although antimicrobial activity has been reported for Diospyros kaki leaves, Agrimoniae Herba, and Polygoni Multiflori Radix, this study emphasized their inhibitory activities against biofilm formation by S. mutans.
In addition, the ability of water-soluble compounds to inhibit biofilm formation was examined in Fig. 3. The result suggests that compounds exhibiting antimicrobial activity may differ from those only inhibiting biofilm formation. Thus, it is necessary to optimize the extraction method in order to maximize the efficacy of the extract compound to antibiotic activity or inhibition of biofilm formation depending on purpose.
Plaque, a type of biofilm formed by S. mutans, adheres to various microorganisms causing periodontal disease and serious health problems (Peterson et al., 2013; Marsh et al., 2015). The importance of inhibiting the formation of such plaques in oral health is well known. Although several studies report agents that prevent plaque formation via their antimicrobial effects (Nakahara et al., 1993; Koo et al., 2002), there have been no reports on compounds without antimicrobial activities that selectively inhibit biofilm formation by S. mutans prior to this study. Although bactericides have the advantage of killing live bacteria, their prolonged use results in the emergence of bacterial resistance (Brauner et al., 2016; Van den Bergh et al., 2016). Therefore, naturally occurring substances that selectively inhibit biofilm formation without killing microorganisms provide a new strategy for improving oral hygiene.
4. CONCLUSION
Nine plant extracts inhibiting biofilm formation of S. mutans were selected from the methanol extract library of 140 plants. Among them, Diospyros kaki leaves, Agrimoniae Herba, and Polygoni Multiflori Radix retained their inhibitory effects even after extraction with water or 50% ethanol. Their water extracts did not affect cell growth but selectively inhibited biofilm formation. Selectively inhibiting biofilm formation without antibiotic activity is different from biofilm control agents in many previous studies. This study suggests the selected plants have new biofilm inhibitors that support their application for oral hygiene improvement.








