1. INTRODUCTION
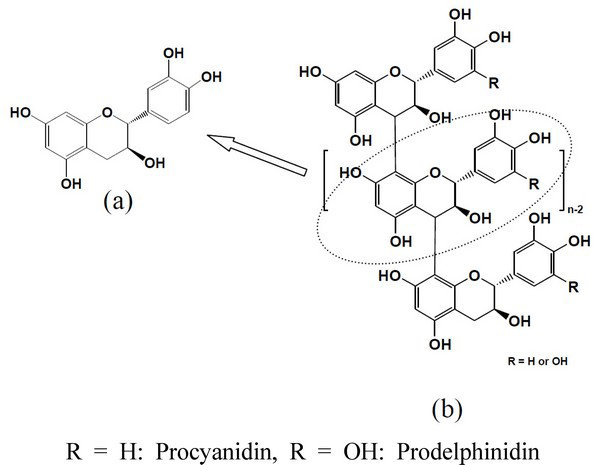
Flavonoids are widely distributed in plants, and considered to be related to a plants resistance to insect and fungal attacks (Schultz et al., 1995; Ohmura et al., 2000; Boue and Raina, 2003; Binbuga et al., 2008; Little et al., 2010). Monomeric flavonoids having antioxidant activity, such as catechin and morin, are known to have good termite feeding deterrence and toxicity at treatment levels of 4% and 6% (Little et al., 2010). Although these flavonoids are reported to have antifeedant activity and toxicity against termites, an effort to commercialize may encounter a resistance because of their relatively high production cost. Procyanidins (PCs) composed of flavan 3-ol subunits (catechin unit) linked mainly through C4-C8 (or C6) bonds (Fig. 1) are a major component of Pinus radiata bark and have a high antioxidant activity (Ku and Mun, 2007). PCs are easily extracted with a polar solvent such as water (Ku et al., 2011), lower alcohols (Li and Maplesden, 1998; Jerez et al., 2006), and weak-alkaline solutions (Mun and Kim, 2009), from P. radiata bark using a simple procedure which may lower the extraction cost. Presently, the product containing PCs as a major compound is selling in the market under the name of pine bark tannin for wood adhesion and the leather industry (Li and Maplesden, 1998).
In this study, we investigated whether PCs-rich extracts such as MeOH extract, HWE, and PCs with high- and low-molecular weight distribution have antifeedant activities against termites in comparison to catechin in order to determine whether they have potential as environmentally- friendly termite control agents.
2. MATERIALS and METHODS
Southern yellow pine wafers measuring 25 mm × 25 mm × 6 mm (R × T × L) were cut from one sapwood lumber board for the termite test. The outer bark of P. radiata was provided from Unid Co. Ltd. in Kunsan, South Korea. The bark was dried in a convection oven at 60 ± 1°C for 48 hours, ground using a Wiley mill equipped with a 1 mm screen. Methanol (MeOH), ethyl acetate (EtOAc), and absolute ethanol (EtOH) were all HPLC grades purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA) and used as extraction and/or desorption solvents to prepare various samples for the termite test from P. radiata bark. Diaion HP 20 was purchased from Mitsubishi Chemical (Tokyo, Japan).
Fifty grams (oven dry weight: o.d.) of pine bark powder and 500 mℓ of MeOH were added to a 1-L Erlenmeyer flask. The flask was then placed in a hood at room temperature for 12 hours with occasional shaking. The slurry was filtered and the residue was washed with 200 mℓ of fresh MeOH. The filtrate and washings were combined and evaporated to reduce the volume to about 150 mℓ. This concentrated MeOH extract was then used in the termite antifeedant activity test. The residue was again washed with 600 mℓ of fresh MeOH and then dried at 130°C in a convection oven for 3 hours. MeOH extract yield was calculated from the decreased weight of the residue after MeOH extraction. The solid content in the MeOH extract was determined and used for calculating the treatment concentration of MeOH extract in the wood wafer.
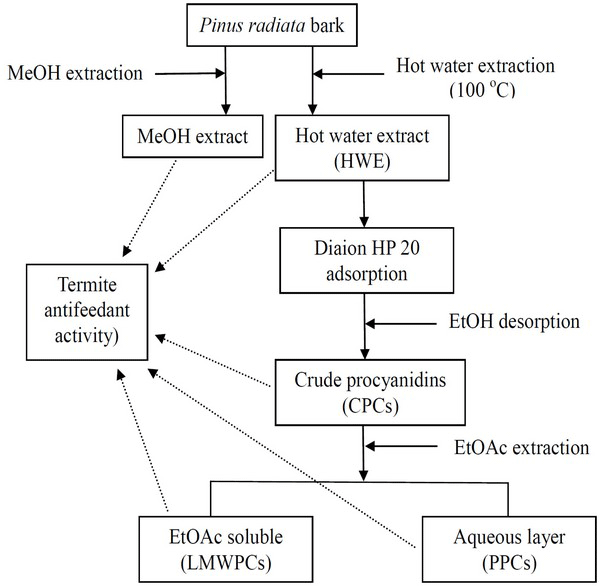
Sixty grams (o.d.) of the bark powder was extracted with 600 mℓ of de-ionized water (DI-water) in a 1-L beaker (liquor to bark ratio of 10). The beaker was then placed on a hot plate and allowed to boil for 1 hour with occasional stirring. The bark residue and HWE were separated by filtration using a 17G3 glass filter. The residue was washed with 600 mℓ DI-water, the filtrate and the washing were combined and used for preparation of crude procyanidins (CPCs), polymeric PCs (PPCs) and low-molecular-weight PCs (LMWPCs). HWE yields were determined in the same manner as the calculation for MeOH extract yield.
Diaion HP 20, commercial adsorbent, was used for the separation of CPCs, PPCs and LMWPCs from HWE as we previously reported (Mun and Kim, 2011; Mun, 2014). Diaion HP 20 (ca. 250 mℓ), fully soaked and washed with EtOH and DI-water, and HWE were transferred to a 2-L Erlenmeyer flask. The adsorption was conducted at room temperature by agitating at 200 rpm for 20 hours. The adsorbent was recovered by filtration and washed with 800 mℓ DI-water and then treated with EtOH to desorb the adsorbed materials onto the adsorbent. The solvent, EtOH, after desorption was removed by evaporation at 60°C and the resulting materials were then vacuum dried overnight at room temperature. We defined this fraction as crude procyanidins (CPCs). The CPCs were dispersed in DI-water and then further extracted with EtOAc. The EtOAc soluble fraction and the aqueous fraction were named LMWPCs and PPCs, respectively. Each fraction was evaporated to dryness at 60°C.
Antioxidant activities of samples prepared for this experiment were dissolved in MeOH and then measured by DPPH (1,1-diphenyl-2-pic-rylhydrazyl radical) free radical scavenging assay, with catechin being used as a positive control. In this method 10 mg of each sample was dissolved in 10 mℓ of MeOH. The sample solution was then diluted to 16.7 μg/mℓ with MeOH. One milliliter of the dilute solution was mixed with 2 mℓ of 0.1 mM DPPH MeOH solution in a test tube and reacted in a water bath at 25°C for 30 minutes. The absorbance was then measured at 518 nm. The results were expressed as catechin equivalent antioxidant capacity.
The samples prepared from this experiment were acetylated prior to conducting gel permeation chromatography (GPC) analysis. For acetylation, 20 mg of each sample was placed in a 10 mℓ vial and dissolved in 2 mℓ of an acetic anhydride-pyridine (1 : 1, v/v) mixture. The acetylation was performed for 48 hours at room temperature. For GPC analysis, 1 mg of the acetylated sample was dissolved in 1 mℓ of tetrahydrofuran (THF) and was subsequently filtered using a syringe filter with a pore diameter of 0.45 μm. The filtrate was then subjected to GPC analysis. A Shimadzu LC-10AD pump unit equipped with a Spectra 100 variable detector (Spectra-Physics, USA) and an AM GEL column (10 mm × 30 cm, American Polymer Standards, Co. Ltd., USA) was used for the experiments. The mobile phase was THF at a flow rate of 0.5 mℓ/min. The molecular weight (MW) calculation was based on a calibration curve obtained using monodisperse polystyrene standards and phenol. The effluent from the column was monitored at 280 nm for the sample and phenol, and at 254 nm for the polystyrene standards.
All of the samples were dissolved in MeOH at a 6% (w/w) level. The wood wafers of predetermined weights were soaked in each sample solution for 15 minutes and then placed in a vacuum oven at 30 mmHg for 15 minutes at room temperature. Sample retentions were calculated from the weight differences before and after sample treatments. After treatment, the wafers were dried at 43°C for 2 hours and then placed in a conditioning chamber (20°C, 12% EMC) for 3 days until constant weights were obtained. MeOH treated wafers was used as controls. Reticulitermes flavipes Kollar termites were employed for the laboratory no-choice tests according to AWPA Standard E1 (AWPA, 2009). After 28 days exposure to the termites, the wood wafers were dried in the same manner as mentioned above to determine the weight loss, and then visually rated using the AWPA E1 scale of 10 to 0. Five replications were conducted for each compound.
3. RESULTS and DISCUSSION
Fig. 2 shows the systematic preparation of MeOH extract, HWE, CPCs, PPCs and LMWPCs from P. radiata bark. The yields of MeOH extract and HWE were 33.6 and 28.9%, respectively, as shown in Table 1. The higher yield of MeOH extract for HWE is likely caused by the high solubility of procyanidins (PCs) in MeOH compared to that in hot water. Previous research reported that MeOH can easily extract PCs, as shown in Fig. 1, out of P. radiata bark (Li and Maplesden, 1998). HWE was soaked in a Diaion HP 20 adsorbent to separate CPCs, which yielded 82.7% of HWE, equating to a 23.9% yield based upon the bark weight. The PCs content determined by UV method (Park et al., 2011) in CPCs was 88%, and they are known to contain a small amount of monomeric flavonoids and some unidentified hydrophobic compounds (Mun and Kim, 2011). CPCs can be separated to high- and low-molecular weight fractions by EtOAc extraction (Ku and Mun, 2007; Mun and Kim, 2011). PPCs and LMWCPs fractionated from EtOAc extraction of CPCs account for 18.1 and 5.5% of the bark weight, respectively (Table 1).
| Yield (% of bark) | ||||
|---|---|---|---|---|
| MeOH extract | HWE | CPCs | PPCs | LMWPCs |
| 32.9 | 28.9 | 23.9 | 18.1 | 5.8 |
All of the samples prepared from P. radiata bark (MeOH extract, HWE, CPCs, PPCs and LMWPCs) were acetylated for the investigation of MW and the results are shown in Table 2. The MeOH extract showed the highest MW and polydispersity. This result indicates that a wide range of MW proanthocysnidins can be easily leached from the bark because of their high solubility in MeOH as mentioned above. The MWs of CPCs and PPCs, separated from HWE, were 3782 Da and 4120 Da, respectively. The polydispersities of both samples (4.6 and 4.2, respectively) were similar to each other. In the case of LMWPCs, since the Mw was 1000 Da or less, it is likely that most of this fraction was composed of dimeric proanthocyanoidins or smaller polyphenols.
| MeOH extract | HWE | CPCs | PPCs | LMWPCs | |
|---|---|---|---|---|---|
| Mw | 6011 | 4281 | 3782 | 4120 | 925 |
| Mn | 807 | 724 | 822 | 973 | 412 |
| Mw/Mn | 7.4 | 5.9 | 4.6 | 4.2 | 2.2 |
All samples prepared except for HWE, which was found to have almost no activity against termites in a preliminary study, were evaluated in the termite antifeedant activity test.
As mentioned above, Little et al. (2010) reported that some monomeric flavonoids having antioxidant activity, such as morin and catechin, showed high termite feeding deterrence and toxicity. Table 3 shows the DPPH free radical scavenging activities of five samples prepared from P. radiata bark (pure PCs, CPCs, PPCs, and LMWPCs) and catechin as a positive control. In spite of the different MW of the aforementioned samples prepared from the bark, which are mainly composed of the catechin unit, they showed similar antioxidant activities to that of catechin. Catechin, flavan 3-ol, as shown in Fig. 1, is known as a potent antioxidant and has strong antifeedant activity against termites (Little et al., 2010). Therefore, we used catechin as a positive control and investigated the effectiveness of the MeOH extract and three different PCs-rich fractions having different MWs in the termite antifeedant activity test. As shown in Table 4, all samples prepared from the bark showed antifeedant activites against termites, but the effectiveness of them was somewhat lower than that of catechin, especially PPCs and MeOH extract which contains higher molecular weight PCs. The antifeedant activity increased in the order of MeOH extract, CPCs, PPCs, and LMWCPs, with LMWCPs exhibiting the highest activity. Although the samples used for the antifeedant activity test are mainly composed of catechin units linked through C4-C8 bonds and have similar antioxidant activities with catechin (Table 3), with the exception of LMWCPs they showed slightly lower antifeedant activities than catechin. The LMWCPs were the most effective deterrent to termite feeding. Ohmura et al. (1999; 2000) reported that larch wood showed a strong antitermite effect because it contains a high amount of the water soluble flavonoid, taxifolin. Some flavonoids isolated from Japanese larch wood, such as taxifolin, quercetin, aromadendrin, naringenin, along with some other monomeric flavonoids and aromatic acids were tested for the antifeedant effect against subtterranean termites. In a previous study, taxifolin, quercetin, and naringenin showed high antifeedant activities. Therefore, it was thought that the LMWCPs has a high antifeedant activity compared to catechin because it contains not only catechin, but also some monomeric flavonoids such as taxifolin and quercetine (Mun and Ku, 2006; 2008). Consequently, the samples containing high amounts of PCs seemed to contribute positively to antifeedant activity, but the effectiveness was somewhat lower than that of catechin. Further study is needed to determine why higher molecular weight PCs showed lower activity against termites. The relationship, if any, between the antifeedant activity and antioxidant activity of the tested PCs-rich extracts and PCs having different molecular weight was not apparent in this study. However, this study showed that antifeedant activity was more dependent on the molecular weight of the PCs than on antioxidant activity.
| Sample | CEAC |
|---|---|
| Catechin | 1.00 |
| Pure PCs | 0.95 |
| CPCs | 1.03 |
| PPCs | 0.94 |
| LMWPCs | 0.96 |
4. CONCLUSIONS
The termite antifeedant activity of PCs-rich solvent extracts and PCs with different MWs prepared from P. radiata bark was investigated. CPCs, PPCs, and LMWPCs showed similar antioxidant activities to catechin, which is the basic unit of PCs used as a positive control. However, antifeedant activity of the PCs against termites was somewhat lower than that of catechin, especially for higher molecular weight compounds. The relationship between antifeedant activity and antioxidant activity of the tested PCs-rich extracts and PCs having different MW was not clear, but the antifeedant activity of PCs appears to be more dependent on their molecular weight than on their antioxidant activity.