1. INTRODUCTION
Wood-decaying fungi are the most destructive agents of wood deterioration worldwide. Although they play a vital ecological role in organic matter decomposition, they cause greater losses to timber structures than those caused by insects, marine borers, and abiotic weathering (Goodell and Jellison, 2008). Wood is widely used globally for furniture production, housing, and construction, as it is lightweight, easy to process, and possesses adequate seismic resistance. However, many tropical species exhibit low natural durability (Jasni, 2016), making them highly susceptible to biodeterioration. Among the main degraders, white-rot fungi, such as Schizophyllum commune, are particularly aggressive, as they degrade both cellulose and lignin, causing up to 30% mass loss in infested wood (Herliyana et al., 2011). Moreover, the extent and pattern of fungal decay vary depending on the wood species and enzymatic activities involved, such as cellulase and laccase production (Ham et al., 2021).
As wood remains an important renewable resource in construction and everyday utilities, the development of safe and sustainable preservation methods is crucial to ensure its durability and long-term service life. Although conventional preservatives such as pentachlorophenol provide effective protection, their synthetic origin raises serious environmental and health concerns (Choudhury et al., 1986). Consequently, research on natural alternatives has gained momentum in line with global trends toward eco-friendly and sustainable wood protection.
Essential oils and plant extracts have emerged as promising candidates due to their broad-spectrum antimicrobial activity, biodegradability, and low toxicity (Chittenden and Singh, 2011; Putri et al., 2025). Several terpenoid-rich oils, including those from Eucalyptus and Cinnamomum trees, have been reported to be effective against wood-decaying fungi, demonstrating their potential for use in bio-based preservation systems (Adfa et al., 2020; Yang et al., 2016). Indonesia possesses abundant natural resources, and its tropical plant species are rich in chemical constituents that can serve as alternative sources of bioactive compounds for managing wood-degrading fungi. For instance, wood vinegar derived from Durio species has been shown to exhibit both antifungal and antitermite activities, supporting its potential as a natural wood protective agent (Suprianto et al., 2023).
Building on this foundation, recent investigations have further emphasized the diverse bioactivities of plant-based compounds, including their antioxidant, antitermite, antimicrobial, larvicidal, and anti-inflammatory effects (Huh et al., 2022; Kuspradini et al., 2024; Oksari et al., 2025; Sari et al., 2025; Yang et al., 2022; Zalsabila et al., 2024). In addition, catechin has been proposed as an alternative protective agent against wood-staining fungi for rubberwood-based products (Nandika et al., 2023). Collectively, these findings underscore the importance of exploring plant-derived bioresources as environmentally friendly and sustainable alternatives to protect wood and other lignocellulosic materials from biological degradation.
Liquidambar excelsa (Noronha) Oken, syn. Altingia excelsa, known as “rasamala” in Indonesia, represents a promising but underexplored source of natural preservatives. The species produces balsam exudates, known as “getah malai” in West Java, Indonesia. Balsam exudates, together with gums and resins, are classified as non-timber forest products and are valuable bioresources obtained from trees without timber harvesting. These exudates are rich in secondary metabolites and secreted from traumatic resin ducts formed in response to wounding or phytohormones (Carolina and Kusumoto, 2020). Methyl jasmonate (MeJA) treatment has been shown to significantly increase resin canal size and exudate yield in conifers, such as Pinus sylvestris and P. radiata (López-Villamor et al., 2021). In our recent studies, we have demonstrated that MeJA treatment effectively induces balsam secretion from wounded L. excelsa branches, whereas wounding alone results in no secretion (Carolina et al., 2024, 2025). Furthermore, we have revealed that the secreted balsam is a complex mixture rich in terpenoids, including ursolic aldehyde, α-pinene, and β-pinene, as well as cinnamates such as cinnamyl cinnamate and phenylpropyl cinnamate (Carolina et al., 2025). The chemicals found in L. excelsa balsam show promise for use as antifungal agents against wood-decaying fungi such as S. commune. However, the antifungal properties of this balsam and the differences among its fractions (ethanol extract, n-hexane extract, hydrosol, and essential oil) have not been studied systematically. Therefore, this study aimed to evaluate the antifungal efficacy of the fractionated L. excelsa balsam components against S. commune using agar plate media. Additionally, the chemical composition of each fraction was determined using gas chromatography–mass spectrometry (GC–MS), and its relationship with the antifungal activity (AFA) was discussed.
2. MATERIALS and METHODS
The procedure used for induction has been described by Carolina et al. (2024). L. excelsa trees with a height of 10–14 m and a diameter at breast height (DBH) of 20–22 cm were selected. Incisions measuring 2–3 cm in length were made on small-diameter (4–6 cm in diameter) branches. Approximately 0.1 mL of a 10% (v/v) solution of MeJA (C13H20O3; Phytotechlab, Lenexa, KS, USA) prepared in Tween-80 (Merck, Darmstadt, Germany) was applied exogenously to the wounded area. Balsam exudates from treated wounds were collected and stored for further processing.
L. excelsa exudate balsam (5.0 g) was subjected to hydro-distillation for 2.0 h in a round-bottom flask containing 200 mL of distilled water using a heating mantle set at 100 ± 2°C. The distillates were collected in an Erlenmeyer flask and separated into two layers: essential oil and hydrosol. The essential oil layer was separated using a separatory funnel and dried over anhydrous sodium sulfate (Merck, Darmstadt, Germany) to remove residual moisture. The remaining aqueous layer (hydrosol) was collected and stored at 4°C.
For solvent extraction, 5.0 g of balsam exudate was refluxed at 60°C for 1.0 h with 50 mL of ethanol (Merck, Darmstadt, Germany; 1:10, w/v) using a reflux condenser. The extract was then concentrated under reduced pressure at 40°C using a rotary evaporator until dryness and stored in sealed amber vials at 4°C. The same procedure was repeated using n-hexane (Merck, Darmstadt, Germany) as the solvent to obtain the hexane extract. The yield (%) of each fraction (ethanol extract, n-hexane extract, and essential oil) was calculated based on the initial balsam weight (5.0 g), using Equation (1) as follows:
The AFAs of L. excelsa balsam fractions were evaluated using the poisoned food technique described by Grover and Moore (1962), with minor modifications. The test solutions consisted of ethanol extract, n-hexane extract, hydrosol, and essential oil, each prepared at the concentrations of 0.5%, 1.0%, 2.0%, 4.0%, and 6.0% (w/v) for the ethanol and n-hexane extracts, and 2.0%, 4.0%, 6.0%, 8.0%, and 10% (v/v) for the hydrosol and essential oil fractions. A mixture of sodium borate Di-sodium tetraborate decahydrate (Sodium borate; Merck, Darmstadt, Germany) and boric acid (Merck, Darmstadt, Germany), adjusted to a boric acid equivalent level of 5.0%, was used as the positive control, and potato dextrose agar (PDA; Thermo ScientificTM OxoidTM, Basingstoke, UK) with Tween 80 was used as the negative control.
Each treatment solution (10 mL) was added to the molten PDA medium and poured into 9.0-cm Petri dishes under aseptic conditions in a laminar flow cabinet. After solidification, one mycelial plug (11 mm in diameter) of S. commune was inoculated at the center of each plate. Cultures were incubated at room temperature, with five replicates per treatment. Colony diameters were measured daily until the mycelium fully covered the positive control plate. AFA was classified into five categories: inactive (0%), weak (0%–25%), moderate (25%–50%), strong (50%–75%), and very strong (> 75%; Mori et al., 1997).
AFA (%) was calculated as described by Mori et al. (1997) using Equation (2) as follows:
where DC represents the control colony diameter (mm), and DT is the treatment colony diameter (mm).
The chemical compounds in the ethanol extract, n-hexane extract, hydrosol, and essential oil were analyzed using GC–MS. The equipment used was an Agilent Technologies (Santa Clara, CA, USA) 7890 gas chromatograph, fitted with an autosampler and a 5975 Mass Selective Detector, equipped with a ChemStation (Dayton, OH, USA) data system. The column used was a 30 m long HP-Ultra 2 capillary column, with an inner diameter of 0.20 mm and a film thickness of 0.11 μm. Helium was used as the carrier gas, with a constant column flow speed of 1.2 mL/min. The initial temperature was set to 80°C, then increased at a rate of 3°C/min to reach 150°C, stabilized for 1.0 min, and further increased at a rate of 20°C/min to reach 280°C, where it was maintained for 26 min. The temperatures at the injection port, ion source, interface, and quadrupole in scan mode were 250°C, 230°C, 280°C, and 140°C, respectively. Electron impact was used as the ionization mode with an ionization energy of 70 eV.
Data were analyzed using two-way analysis of variance (ANOVA) to evaluate the effect of the two factors in a completely randomized design. The first and second factors consisted of four different fractions, i.e., ethanol extract, n-hexane extract, hydrosol, and essential oil, and five different concentrations of each fraction, respectively. The test was performed using IBM SPSS Statistics (Statistical Package for Service Solutions; IBM, Armonk, NY, USA) version 26.0.
3. RESULTS and DISCUSSION
Balsam exudates of L. excelsa were generally observed 14 days after the application of 10% MeJA, which is a transparent sticky resin with a characteristic aroma [Fig. 1(b)]. Naturally exuded L. excelsa balsam was not available during the experimental period because spontaneous exudation in this species occurred irregularly and in extremely small quantities. Balsam is typically secreted in response to environmental or physiological stress, and naturally exuded amounts are insufficient for analytical or bioassay purposes. Therefore, MeJA induction was necessary to stimulate and increase the exudate yield for chemical and antifungal evaluations.
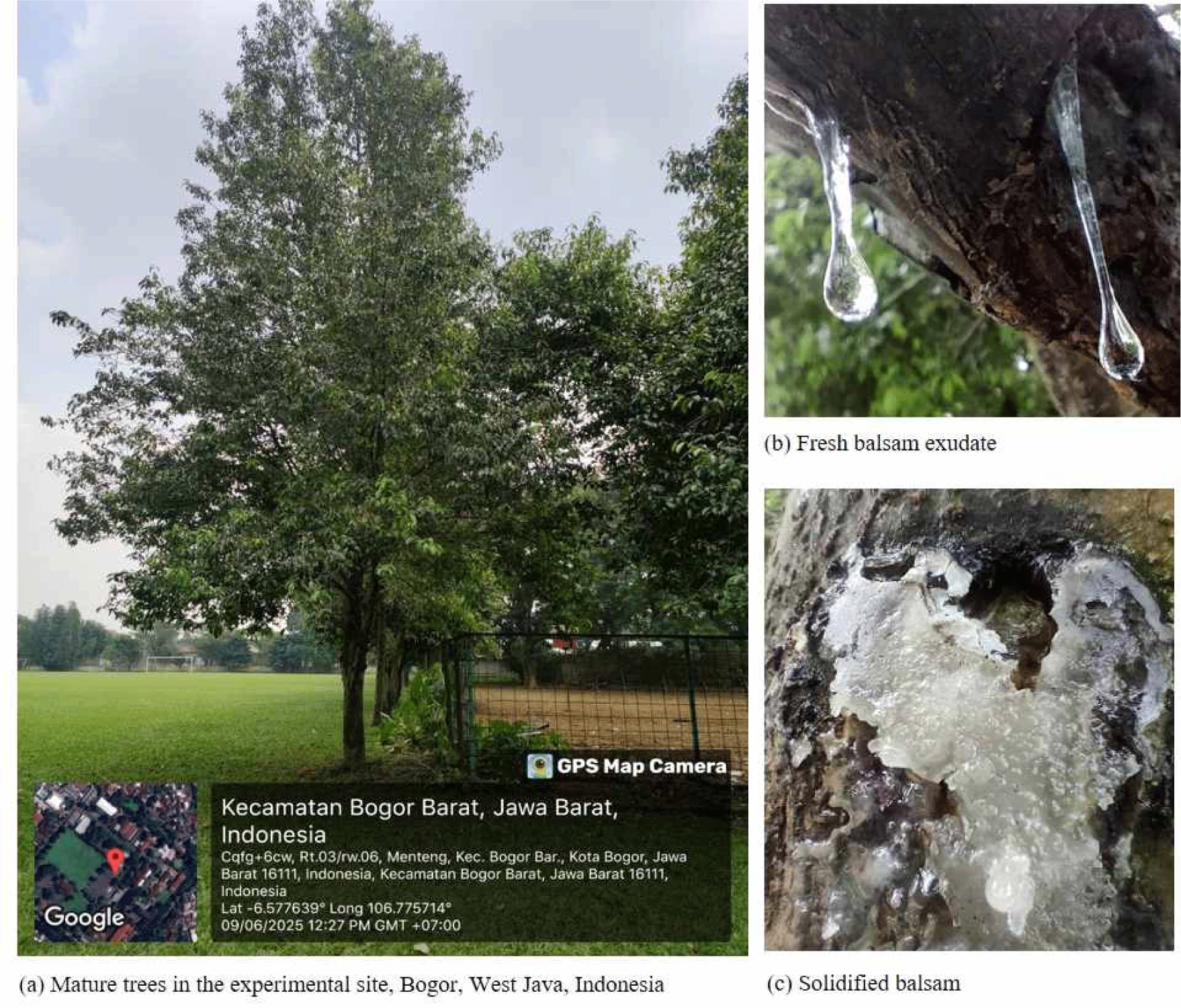
The 10% MeJA concentration was selected based on our previous findings that the 10% MeJA treatment results in the highest balsam yields compared with those obtained using lower MeJA concentrations or other stimulant treatments (Carolina et al., 2024, 2025). This observation in L. excelsa is consistent with previous reports on other woody plants, in which jasmonate application has been shown to elicit comparable defense responses. For instance, Hudgins et al. (2004) demonstrated that MeJA induces the formation of traumatic resin ducts and enhances resin flow in P. taeda, whereas Martin et al. (2003) provided evidence that MeJA triggers both the formation of traumatic resin ducts and the accumulation of terpenoid resins in the developing xylem of Picea abies. Similarly, Saniewski et al. (2004) described the jasmonate-mediated stimulation of gum and resin secretion in stone fruit trees, and Yamamoto et al. (2020) confirmed that jasmonate signaling plays a central role in the regulation of latex and resin exudation in tropical tree species. Together, these studies reinforce the interpretation that MeJA acts as a universal elicitor of resin-type secretions across diverse taxa. Our findings extend this concept to L. excelsa, where wounding and high concentrations of MeJA are particularly effective in promoting balsam exudation.
The production of resin and balsam exudates is influenced by both internal and external factors. Internal factors such as tree diameter, height, and physiological conditions have been shown to correlate strongly with resin yield. For example, Rodríguez-García et al. (2014) demonstrated that a larger diameter and higher percentage of live crowns significantly increased resin yield in P. pinaster, whereas Lukmandaru et al. (2024) reported that trees with greater resin duct density and duct diameter tended to have higher resin yields. Externally, factors such as the wounding method (width of wounds and number of faces tapped), orientation of tapping, and environmental conditions (climate, elevation, and seasonality) also play major roles. Studies in P. pinaster have shown that wound width and the number of tapped faces strongly affect resin output, and that production peaks vary by season and location (García-Méijome et al., 2023). Similarly, in P. oocarpa, DBH, tree height, number of tapped faces, and soil/ climate type correlate positively with resin yield (Reyes-Ramos et al., 2019). Finally, studies on P. merkusii indicated that altitude, temperature, humidity, and soil type modulate the response to tapping and stimulants (Lukmandaru et al., 2021), confirming that the environmental context is critical in resin exudation studies. Therefore, these internal and external factors should be considered when optimizing balsam production using L. excelsa.
During collection, the induced balsam appeared clear and viscous, but its color and texture gradually changed from transparent and sticky to pale yellow and solidified, respectively [Fig. 1(c)]. Similar color changes have been reported in MeJA-treated L. excelsa balsam, shifting from colorless to whitish or pale yellow over time at room temperature (Carolina et al., 2024). Such changes are commonly observed in gums, resins, and essential oils, and are typically attributed to the evaporation of volatiles, oxidative reactions, and the presence of unstable minor constituents. For example, essential oils from Mentha × piperita, M. spicata, Origanum vulgare, and Thymus vulgaris stored at moderate to high temperatures gradually shift in color from pale yellow to more intense yellow/orange owing to the oxidation of sesquiterpenes and the loss of volatile monoterpenes (Ganosi et al., 2023). Similarly, gum rosin and resin acids exposed to light or stored under ambient conditions show darkening and increased viscosity owing to the oxidative polymerization of terpenoid acids and the formation of chromophoric degradation products (Liu et al., 2020).
The reflux extraction yielded an ethanol extract of L. excelsa balsam exudates at 63.86% and an n-hexane extract at 63.55%, whereas hydro-distillation produced an essential oil at 19.05% as the volatile fraction (Table 1). The extraction and distillation processes were conducted separately using equal portions (5.0 g) of balsam for each treatment. Thus, the yield values represent the comparative efficiency of each method rather than the cumulative yields from a single batch. The presence of substantial amounts of ethanol and n-hexane extracts indicated that L. excelsa balsam contained a broad spectrum of polar to non-polar metabolites. Comparable yields have been reported in resinous materials from other Liquidambar species, where both alcoholic and hydrocarbon solvents have been used to recover high proportions of triterpenoids, resins, and aromatic constituents (Çetinkaya et al., 2022).
| Sample | Yield (%) |
|---|---|
| Ethanol extract | 63.86 |
| n-Hexane extract | 63.55 |
| Essential oil | 19.05 |
Hydro-distillation also produced hydrosols as a co-product, representing the aqueous fraction containing dispersed volatile constituents. Although hydrosols are typically obtained in larger amounts than those obtained for essential oils and have historically been undervalued, recent studies have highlighted their potential as a valuable bioactive fraction. Hydrosols contain biologically active volatiles and phenolic compounds and can serve as natural preservatives (Almeida et al., 2024). Similarly, Khalaf and Zahra (2020) reported that hydrosols from Eucalyptus camaldulensis displayed antibacterial activity, including activity against biofilm-forming bacteria, although they were somewhat weaker than that of the essential oil in some test systems.
The physical characteristics of the essential oil and hydrosol fractions are summarized in Table 2. The essential oil fraction exhibited a pale yellow, clear appearance, strong balsamic odor, low specific gravity (0.8397 at 27°C), and a refractive index of 1.468 at 20°C, which are consistent with the properties of volatile terpenoid-rich oils obtained from other hardwood resins. In contrast, the hydrosol fraction was clear and transparent, with a weaker balsamic aroma, slightly higher specific gravity (0.8537 at 27°C), and a lower refractive index (1.341 at 20°C) than those recorded for the essential oil fraction.
The GC–MS profiling of L. excelsa balsam fractions revealed a diverse array of terpenoids and aromatic compounds, with clear differences between hydrodistillation and reflux extraction (Table 3). The essential oil fraction was dominated by γ-terpinene (40.60%), α-pinene (25.09%), and β-pinene (14.77%), confirming that the volatile components of L. excelsa are primarily monoterpenoids rather than aromatic compounds.
Compared to published data for other Liquidambar species, both similarities and distinctions were evident. For example, α-pinene, β-pinene, and β-caryophyllene have been frequently identified as major constituents in L. formosana (DeCarlo et al., 2020), whereas styrene and α-pinene occur predominantly in L. orientalis (Gurbuz et al., 2013; Hafizoglu et al., 1996). In addition, previous studies on L. styraciflua leaves and stems have consistently reported α-pinene, β-pinene, and d-limonene as the dominant volatile constituents (El-Readi et al., 2013; Zhao et al., 2025). These comparisons indicate that L. excelsa shares some terpenoid features with other Liquidambar species, particularly the prominence of α-pinene and β-pinene, yet its overall profile is not identical to any previously reported Liquidambar species. The partial overlap, combined with species-specific differences, suggests that L. excelsa balsam may have distinct functional properties and potential applications compared to other Liquidambar sources.
In contrast, the hydrosol fraction contained a higher proportion of oxygenated terpenes and aromatic alcohols, particularly terpinen-4-ol (24.83%) and 3-phenylpropanol (5.95%). Such an enrichment of more polar and water-dispersible constituents is consistent with previous findings on hydrosols from hardwoods, such as Cupressus leylandii, in which oxygenated terpenoids were preferentially partitioned into the aqueous phase (Almeida et al., 2024).
Organic solvent extracts were characterized by a predominance of pentacyclic triterpenoids and aromatic esters. Ursolic aldehyde (22%–23%) and lupeol (8%–13%) were the principal triterpenoid constituents, whereas cinnamyl cinnamate (styracin, 8%–13%) was detected as the major aromatic ester. The detection of additional triterpenoids such as uvaol (6.38%) further corroborated the resinous character of these extracts. These compositional features are consistent with earlier analytical studies on Liquidambar balsams that reported pentacyclic triterpenes and cinnamate esters as characteristic markers of Storax/balsam exudates (Custódio and Veiga-Junior, 2012; Hafizoglu et al., 1996). L. excelsa balsam displayed specific chemical traits, such as the abundance of ursolic aldehyde and lupeol. The prominence of triterpenoids in L. excelsa corresponds well with the chemotaxonomic observations of Courel et al. (2019), who highlighted triterpenoid profiles as key diagnostic traits for differentiating genuine balsams.
Notably, our results are consistent with the ethanol extract profile reported by Carolina et al. (2025), who identified ursolic aldehyde, lupeol, and cinnamyl cinnamate as the dominant components of L. excelsa balsam. The overlap in major constituents across these independent studies suggests that the ethanol-soluble fraction of L. excelsa exhibits a stable chemical signature, although minor constituents may vary depending on environmental conditions or extraction methods.
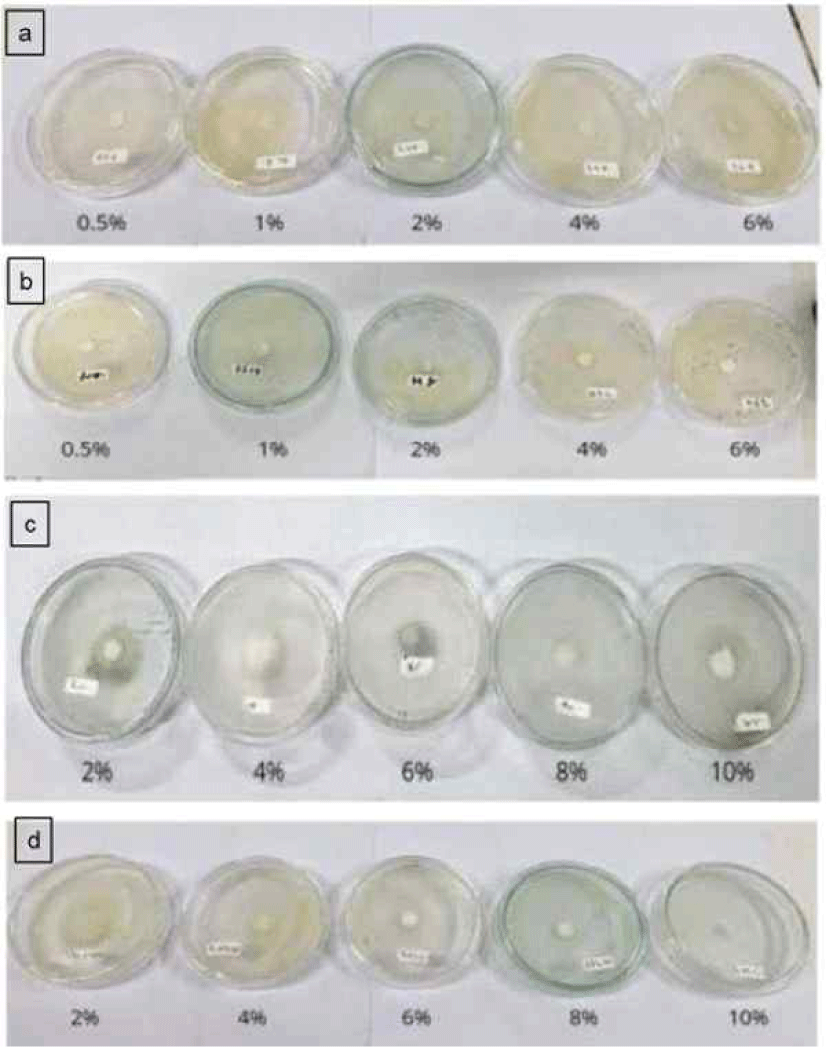
Bioassays against S. commune revealed that all fractions of L. excelsa balsam extract inhibited fungal growth in a concentration-dependent manner (Table 4, Fig. 2). The essential oil and hydrosol were the most effective, achieving complete inhibition at a concentration of 10%, whereas the ethanol and n-hexane extracts produced strong inhibition at a concentration of 6.0%. At the same concentration, the AFAs of the essential oil and hydrosol extracts were higher than those of the ethanol and n-hexane extracts. Two-way ANOVA confirmed the significant effects of both the fraction and concentration, whereas their interaction was not significant (Table 5).
| Source of variation | df | F-value | p-value |
|---|---|---|---|
| Fraction | 3 | 151.604 | 0.000 |
| Concentration | 2 | 56.864 | 0.000 |
| Fraction × concentration | 6 | 0.480 | 0.820 |
The GC–MS analysis revealed γ-terpinene, α-pinene, and β-pinene as dominant in the essential oil, while the hydrosol was enriched in terpinen-4-ol and 3-phenylpropanol (Table 3). This suggests that the monoterpenoids effectively diminished the growth of S. commune. In contrast, solvent extracts exhibited lower inhibition. They contain triterpenoids such as ursolic aldehyde and lupeol and aromatic cinnamate esters such as cinnamyl cinnamate (Carolina et al., 2025). Similar chemical profiles have been reported in Liquidambar balsams with antimicrobial and cytotoxic activities (Carolina et al., 2025; Courel et al., 2019), suggesting that these non-volatile-rich fractions may exert slower or complementary antifungal effects.
4. CONCLUSIONS
The results obtained in this study provide the first evidence of the AFA of L. excelsa balsam fractions against S. commune, a common wood-decaying basidiomycete. The GC–MS analysis revealed that L. excelsa balsam was dominated by mono- and triterpenoids, as well as cinnamate esters. These volatile components were found to suppress fungal growth more effectively, suggesting their potential as eco-friendly antifungal agents for wood protection. These findings will contribute to the sustainable utilization of forest products. However, even when applied as wood preservatives, the volatile components evaporate over time, reducing their concentration. Therefore, further studies are needed on the durability of the preservative effects and the development of effective wood treatment methods. In addition, to maintain the quality of L. excelsa balsam, further research is required on the seasonality and genetic factors that affect its chemical composition.
