1. INTRODUCTION
Litsea, one of the largest genera in the Lauraceae family, is an important group of vegetation is widely distributed across tropical and subtropical forests (Jose et al., 2015; Tanaka et al., 2009; Wong et al., 2014). Species in this genus exhibit characteristic aromatic profiles and have long been used in traditional medicine to treat various conditions such as headaches, inflammation, intoxication, rheumatism, diarrhea, dysentery, spasms, wounds, fever, infections, bleeding, colic, vomiting, and other ailments (Chang and Chu, 2011; del Carmen Jiménez-Pérez et al., 2011; Hosamath, 2011; Rath, 2005; Yusuf et al., 2009).
Litsea species are also rich in bioactive compounds, including flavonoids (Lee et al., 2005; Wang et al., 2010), butanolides (Cheng et al., 2001, 2010), lignans (Pan et al., 2010), sesquiterpenes, fatty acids (Agrawal et al., 2011; Zhang et al., 2003), 1,3-diarylpropan-2-ol (Zhao et al., 2003), coumarins, syringaldehyde, and essential oils (Amer and Mehlhorn, 2006; Chowdhury et al., 2008; Guzmán-Gutiérrez et al., 2012). Essential oils from Litseaacutivena and Litseacubeba have demonstrated notable antibacterial and antifungal activity (Ho et al., 2011; Luo et al., 2004; Su and Ho, 2016). Various Litsea species exhibit antibacterial (Li et al., 2014; Wong et al., 2014), antifungal (Cruz et al., 2014; Pambudi et al., 2018), antioxidant (Arfan et al., 2008; Wulandari et al., 2018), cytotoxic (Feng et al., 2009), and anti-inflammatory properties (Gogoi et al., 2018).
Wang and Liu (2010) reported that various parts of L. cubeba, including the fruits, leaves, roots, buds, stems, and flowers, can produce essential oils rich in citral (B), β-phellandrene, and β-terpinene. The leaf oil of this species shows strong bactericidal effects against Escherichia coli (Nguyen et al., 2017) and antimicrobial activity against cariogenic bacteria such as Streptococcus mutans, Streptococcus sobrinus, and Streptococcus sanguinis (Yang et al., 2013). Similarly, the leaf oil of Litseapetiolata inhibits several pathogenic bacteria, including Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae, Vibrio cholerae, Vibrio parahaemolyticus, and Salmonella typhimurium (Thongthip et al., 2017). Ho et al. (2011) also found that the leaf and root oils of L. acutivena and the leaf oil of L. coreana possess strong antimicrobial and anti-wood rot activities. In addition, the leaf and bark extracts of Litsea glutinosa exhibited antibacterial activity against S. aureus, S. epidermidis, E. coli, Enterobacter intermedium, and Salmonella spp. (Haque et al., 2014).
Litsea angulata is native to Borneo, Sumatra, Java, and New Guinea and is commonly found in the forests of East Kalimantan. In South Korea, it is traditionally used to treat boils and inflammation (Ariyani and Mustikasari, 2010). Previous studies reported that seed extracts of L. angulata contain alkaloids and tannins and have potential as natural spermicides (Akmal et al., 2016). Saputri and Susiani (2018) found that the fruits and seeds of L. angulata exert antioxidant activity. Kuspradini et al. (2019) demonstrated that the bark, branches, and leaves of this species are rich in natural antioxidants and possess antibacterial properties. Crude essential oils extracted from the leaves of L. angulata also show antimicrobial activity against S. mutans, S. sobrinus, S. aureus, and Candida albicans (Kuspradini et al., 2020). In contrast to previous studies using crude extracts, we employed two different distillation techniques, simple fractional (SF) and spinning band (SB) distillation, to separate and isolate specific fractions of the essential oil. This approach enables a more precise understanding of the bioactive constituents that contribute to antibacterial efficacy.
Despite advancements in medicine, microbial infections, including those caused by bacteria, viruses, parasites, and fungi, remain important public health issues in many countries (Jawetz et al., 2005). Among these, dental caries is one of the most prevalent infectious diseases worldwide (Chaiya et al., 2013; Forssten et al., 2010), greatly contributing to the burden of oral health problems (Widayati, 2014). Dental caries typically develop when food residues adhere to teeth, promoting calcification, decay, and eventual tooth damage (Sinaga, 2013). The main causative agents include lactic acid bacteria, particularly S. mutans and Lactobacillus spp.
Although lactic acid bacteria support digestive health, they can contribute to tooth decay by fermenting dietary carbohydrates and producing acids that erode enamel. These bacteria become active shortly after consumption, forming biofilms and plaques. To prevent these actions, topical antimicrobial agents such as mouthwashes and toothpaste are used. Synthetic antibiotics, including penicillin, vancomycin, and chlorhexidine, can lead to side effects, such as antibiotic resistance and dental discoloration (Van Winkelhoff et al., 2000).
In response to these concerns, natural antibacterial compounds are being increasingly evaluated as safer alternatives. Plant-derived essential oils are particularly attractive because of their low risk of adverse effects (Indrawati et al., 2017).
Studies of the antibacterial potential of L. angulata essential oil, particularly its individual chemical fractions, are limited. Therefore, this study was conducted to (1) fractionate L. angulata essential oil using both simple SF and SB distillation, (2) identify the chemical constituents of each fraction, and (3) evaluate and compare their antibacterial activities against oral pathogens. This approach allows for detailed analysis of how the fractionation method influences chemical composition and bioactivity.
2. MATERIALS and METHODS
Fresh leaves of L. angulata were collected from the Educational Forest (KHDTK Lempake), Faculty of Forestry and Tropical Environment, Mulawarman University, Samarinda, East Kalimantan, Indonesia (00°26’55.1”S, 117°12’36.2”E). The experiments were conducted in 2018 at the Forest Product Chemistry and Renewable Energy Laboratory, Faculty of Forestry and Tropical Environment, Mulawarman University. A specimen of the plant was deposited as a voucher in the Dendrology Laboratory of Mulawarman University.
Analytical-grade chemicals such as nutrient broth, glucose, and anhydrous sodium sulfate were purchased from Merck (Darmstadt, Germany). All other reagents used were commercial standards.
Essential oils were extracted via water and steam distillation from 4,200 g of fresh L. angulata leaves. The leaves were air-dried for 1 day. Fractionation was performed at distillation time intervals of 0–60 min (SF1), 60–120 min (SF2), and 120–180 min (SF3) as described by Zheljazkov et al. (2014) with slight modifications.
At the end of each interval, the oil fractions were separated from the water, and any remaining moisture was removed using anhydrous sodium sulfate. The yield of each fraction was calculated as the percentage of the oil volume relative to the dry weight of the plant material. The essential oils were stored in sealed vials in the dark until further analyses.
The essential oil from L. angulata leaves was extracted using steam distillation method (Kuspradini et al., 2016; Putri et al., 2018). The samples were air-dried at room temperature for one day. The essential oils were prepared for further analyses. The leaf oil was fractionated using the SB distillation method with a B/R Instrument-Spinning Band Distillation System Model 36-100 (Amrullah et al., 2017). Approximately 50 mL (43.88 g) of essential oil was added to a boiling flask connected to a fractionating column 90 cm in length and controlled by a heat mantle and condenser system (35°C). This process was maintained under vacuum conditions using a vacuum pump with the following settings: pressure 10 mmHg, initial heat 24%, equilibration time 15 min, maximum pot temperature 300°C, and heat rate 16%. The fraction was separated according to the boiling points (60°C–300°C). The volume of various fractions is presented as a percentage of the yield (% w/w; Torres et al., 2012).
The chemical composition of each oil fraction was analyzed using gas chromatography-mass spectrometry (GC-MS; QP-2010, Shimadzu, Kyoto, Japan) equipped with an RTX-5MS capillary column (30 m × 0.25 mm × 0.25 μm). The column conditions were as follows Iordache et al. (2011): injector temperature of 250°C, detector temperature of 280°C, inlet pressure of 93.5 kPa, split ratio of 200:1, column flow rate of 1.44 mL/min, linear velocity of 49.3 cm/s, and helium as a carrier gas.
Compounds were identified by comparing their mass spectra with the NIST database and by calculating retention indices (RI) using C8–C20 alkane standards as described by van Den Dool and Dec. Kratz (1963). Each analysis was performed in duplicate.
The essential oil fractions were screened for their antibacterial activity using the agar well diffusion method, according to the method described by Kuspradini et al. (2018). The bacterial strains used were S. mutans (KCCM 40105), S. sobrinus (KCCM 11898), S. aureus, and Lactobacillus casei. Briefly, the plates were inoculated with the bacterial suspension under aseptic conditions; the 7-mm diameter wells were filled with 20 μL of the samples. The agar plates were incubated at 37°C for 18–24 h (Su et al., 2015). The turbidity of the bacterial suspension was measured at 600 nm (Jahangirian et al., 2013). Chloramphenicol [K(+)1] and chlorhexidine [K(+)2; concentrations of 0.001% or 0.01 mg] were used as standard antibiotics for the positive control, and 40% ethanol was used as the negative control. The concentrations of essential oil used in this test were 100%, 50%, and 25% measured in 20 μL of oil and converted to mg (mg/20 μL) as shown in Table 1. The study was performed in triplicate. After incubation, the diameter of inhibition of bacterial growth was observed and measured in millimeters using a ruler. The data are presented as the average of three replicate experiments.
3. RESULTS and DISCUSSION
Fractional distillation separates essential oils into several fractions. During this process, some variables can be used as treatments to increase the quality of the oil (Amrullah et al., 2017). The aim of this study was to separate L. angulata leaf essential oils into distinct fractions to enhance the oil quality. Two methods were employed: SF distillation (based on distillation time) and SB distillation (based on boiling point ranges).
SF distillation was performed using the water and steam distillation method for different distillation times: 0–60 min (SF1), 60–120 min (SF2), and 120–180 min (SF3). The air-dried material (4,200 g) prepared one day prior as described in section 2.2 was used as the raw material. The yield was measured based on the distribution of the fraction weight with the raw material weight (oven dry/OD), which was multiplied by 100 to obtain the percentage yield.
SB distillation was performed based on the boiling points of the main components of the pure oil. L. angulata leaves were distilled to extract essential oil, which was then fractionated using an SB tool. The essential oil (± 50 mL, 43.88 g, 1.05%) was subjected to the oil distillation process three times, with each process lasting for ± 3–4 h. The yield of each fraction was measured based on the relationship between the fraction weight and essential oil weight (w/w), which was then converted to the initial oil yield weight (Torres et al., 2012).
Fractional distillation of L. angulata leaf essential oil with SF and SB distillation yielded three oil fractions. The fractions produced by each method had various yields (%) and colors, as presented in Table 2.
The results of the SF distillation showed that the highest yield was obtained in fraction SF1 (0.83%), whereas the lowest yield was obtained in fraction SF3 (0.07%; Table 2). This approach was used to isolate essential oil into fractions in a simple manner. This method is effective for obtaining L. angulata oil quickly, as the highest yield was obtained from the first distillation, followed by a second fraction within 60–120 min; thus, the entire process was completed in 0–120 min. Water and steam distillation is a simple method for obtaining essential oils in a short distillation time, high yield, and high quality. This distillation system is widely used because of the low potential for oil decomposition (ester hydrolysis, polymerization, resinification; Qin et al., 2014; Stratakos and Koidis, 2015; Turek and Stinzing, 2013).
The fractionation results from SB distillation in Table 2 show that the oil did not separate between temperatures of 60°C–160°C but did separate in the temperature range of 160°C–220°C. The SB6 fraction showed the highest yield (0.53%), followed by SB5 (0.11%), and SB7 (0.08%). During this process, residual non-distilled materials (SB8) remained in a paste form, even at the highest temperature. According to Savakar et al. (2017), SB distillation involves the use of a metal column to isolate samples with a broad range of boiling points. The temperature determined during the distillation process is the component distillation point; thus, the distillation temperature range matched the boiling point of the constituent material components. Using higher temperatures leads to decomposition and resinification of the produced distillate (Amrullah et al., 2017; Mangun et al., 2005).
Visual observation of the oil fraction of L. angulata showed that the color of oil produced using each method varied. The oil first obtained by SB fractional distillation exhibited a different color than that observed in the test results, as the fractions were oxidized quickly, resulting in a color change. Fraction SB7 was orange in color, possibly because of its high boiling point (210°C–220°C); therefore, the SB method is not recommended for samples with high boiling points to ensure good color quality. The color variation of the fractionated oil may reflect differences in the chemical composition, particularly the content of volatile compounds such as monoterpenes and sesquiterpenes (Amrullah et al., 2017). Supriono and Susanti (2014) observed that darker patchouli oil tended to have a lower alcohol content, in contrast, in our study, fraction SB6—despite its darker color—contained a high proportion of α-phellandrene and showed strong antibacterial activity. Essential oils frequently appear clear and fluid; however, some oils, such as Orris, are solid, whereas others, such as guaiac wood, are semi-solid at room temperature. Most essential oils are either colorless or pale yellow; however, some oils, such as blue chamomile and European valerian, exhibit deeper colors, with the latter being green (Tisserand and Young, 2013). Factors such as the drying of raw materials and extraction procedure may influence the essential oil color (Ahmad et al., 2014; Rahimmalek and Goli, 2013).
The constituent chemical components of each oil fraction of L. angulata obtained from both fractional distillation methods were analyzed using GC-MS. GC-MS analysis is a rapid and accurate method for separating complex mixtures, analyzing mixtures in small amounts, and obtaining data on the structure and identity of organic compounds (Agusta, 2000). Because essential oils contain volatile compounds, they can be analyzed using GC-MS. The Kovats retention index value (RI) of the identified compounds was compared with the reference RI in NIST to confirm the results of compound identification, and the RI was calculated by comparison with the alkane standard (C8–C20).
The constituent compounds identified in each fraction of L. angulata leaf essential oil using SF and SB distillation are shown in Tables 3 and 4. Based on the GC-MS results, each oil fraction of L. angulata contained different main constituent compounds, although the dominant compounds were the same between SF and SB distillation, and some compounds were present in samples obtained using both methods.
The chemical compounds identified in fractions SF1–SF3 are shown in Table 3. Based on the GC-MS data, the dominant compounds in fraction SF1 were monoterpenes, with main compounds including α-phellandrene (47.97%), acetic acid 1,3,3-trimethyl-2-oxa-bicyclo[2.2.2]oct-6-yl ester (10.25%), and β-cymene (6.13%). The two dominant chemical components in fraction SF2 were monoterpenes (42%) and sesquiterpenes (33.36%). The four main compounds were α-phellandrene (15.22%), acetic acid 1,3,3-trimethyl-2-oxa-bicyclo[2.2.2]oct-6-yl ester (12.56%), trans-caryophyllene (11.92%), and (-)-caryophyllene oxide (10.07%). Fraction SF3 was dominated by sesquiterpenes, as well as trans-caryophyllene (31.43%), (-)-caryophyllene oxide (12.74%), and α-copaene (8.98%).
The distillation time can also affect the quantity and quality of the essential oil. The duration of essential oil extraction significantly influences both the yield and proportion of chemical compounds present in the oil (Oliveira et al., 2017). Chatzopoulou and Katsiotis (1995) and Prins et al. (2006) evaluated the influence of extraction time on the chemical composition of essential oils and detected the concentration of oxygenated compounds at the beginning of the extraction process. The chemical components and amounts contained in the distillation results were diverse. Low-boiling point components such as α-pinene, camphene, β-pinene, and myrcene were present at higher concentrations at the beginning of the distillation but decreased over time, reaching a minimum at the end of distillation (Zheljazkov et al., 2014). Other compounds in the sesquiterpene groups in essential oils were found in greater amounts and required longer distillation times (up to 5 h) because the molecules were non-polar (Oliveira et al., 2017).
Table 4 presents the GC-MS data for the oil fraction obtained using SB distillation. The chemical compounds identified in fraction SB5 were dominated by hydrocarbon monoterpene (43.84%), oxygenated monoterpene (33.65%), oxygenated sesquiterpene (1.58%), and other compounds (0.99%). Three main compounds in fraction SB5 were α-phellandrene (29.28%), p-cymene (7.53%), and 1.3.3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol (7.65%). The main constituents of this fraction are classified as hydrocarbon monoterpene and oxygenated monoterpene compounds. GC-MS analysis showed that the main compounds in fraction SB6 consisted of three peak compound components included in hydrocarbon monoterpene family, namely α-phellandrene (21.44%), p-cymene (7.87%), and oxygenated monoterpene, specifically ascaridole (8.64%). Fraction SB7 was dominated by sesquiterpene group compounds (66.56%) followed by oxygenated monoterpenes (20.09%), other compounds (5.42%), and hydrocarbon monoterpenes (5.80%). Three main components of peak compounds in this fraction were α-copaene (26.13%), β-bisabolene (12.47%), and δ-cadinene (9.79%), which are all members of the sesquiterpene group.
Fractional distillation is used to separate crude oil into useful substances (or fractions) containing different hydrocarbons at different boiling points. A crude oil fraction with a higher boiling point has a larger number of carbon atoms, higher molecule weight, darker color, and lower viscosity compared to these factors in fractions with lower boiling points (Kumar and Tripathi, 2011). Maiwald and Schwantes (1991) isolated essential oils from Curcuma rhizomes based on their boiling points. Compounds in the essential oil contain monoterpenes with boiling points of 140°C–180°C, such as 1.8-cineol and α-phellandrene, and sesquiterpenes with boiling points of > 200°C, such as β-bisabolene. α-Phellandrene is a natural compound that can stimulate the immune response in leukemia model rats (Lin et al., 2014). Additionally, α-phellandrene exerts effects against human breast cancer and prostate tumor cells (Essien et al., 2012). Monoterpenes [such as eucalyptol, borneol, camphor, bornyl acetate, carvacrol, menthol, γ-terpinene, (+)-α-pinene, (-)-β-pinene, and p-cymene] are pivotal components of essential oil produced through liquid extraction and distillation with steam from edible plant species and medicinal plants (Crozier et al., 2006).
L. angulata crude essential oil and its fractions were evaluated for their antibacterial activity in vitro using the agar well diffusion method. This method is based on the antibacterial abilities of compounds, which influence the diameter of the inhibition zone around the test bacteria (Nurainy et al., 2008). In most cases, the Standard Deviation (SD) values were relatively small, indicating good reproducibility of the results. However, some treatments showed larger SD values, suggesting variability in the bacterial response or sample sensitivity. In this study, two types of antibiotics were used as positive controls: chloramphenicol and chlorhexidine. Chloramphenicol is an antimicrobial agent that can be used to treat cocci and basil gram-positive bacteria, as well as aerobic and anaerobic gram-negative gram bacteria (U.S. Department of Health and Human Services, 2000). Chlorhexidine is among the most common and widely used antiplaque and anti-gingival agents (Balagopal and Arjunkumar, 2013; Mathur et al., 2011). The inhibition activity of every L. angulata oil fraction at concentrations of 100%, 50%, and 25%, which were converted to mg/20 μL, against the four test bacteria was determined by measuring the clear zone. The results indicated that the oils exerted antibacterial activities. The inhibition diameters created by the L. angulata essential oil fractions against S. mutans, S. sobrinus, S. aureus, and L. casei compared to those created by the crude oil and synthetic standards are presented in Fig. 1. Each fraction obtained using the SF distillation method exhibited antibacterial activities against these four bacteria.
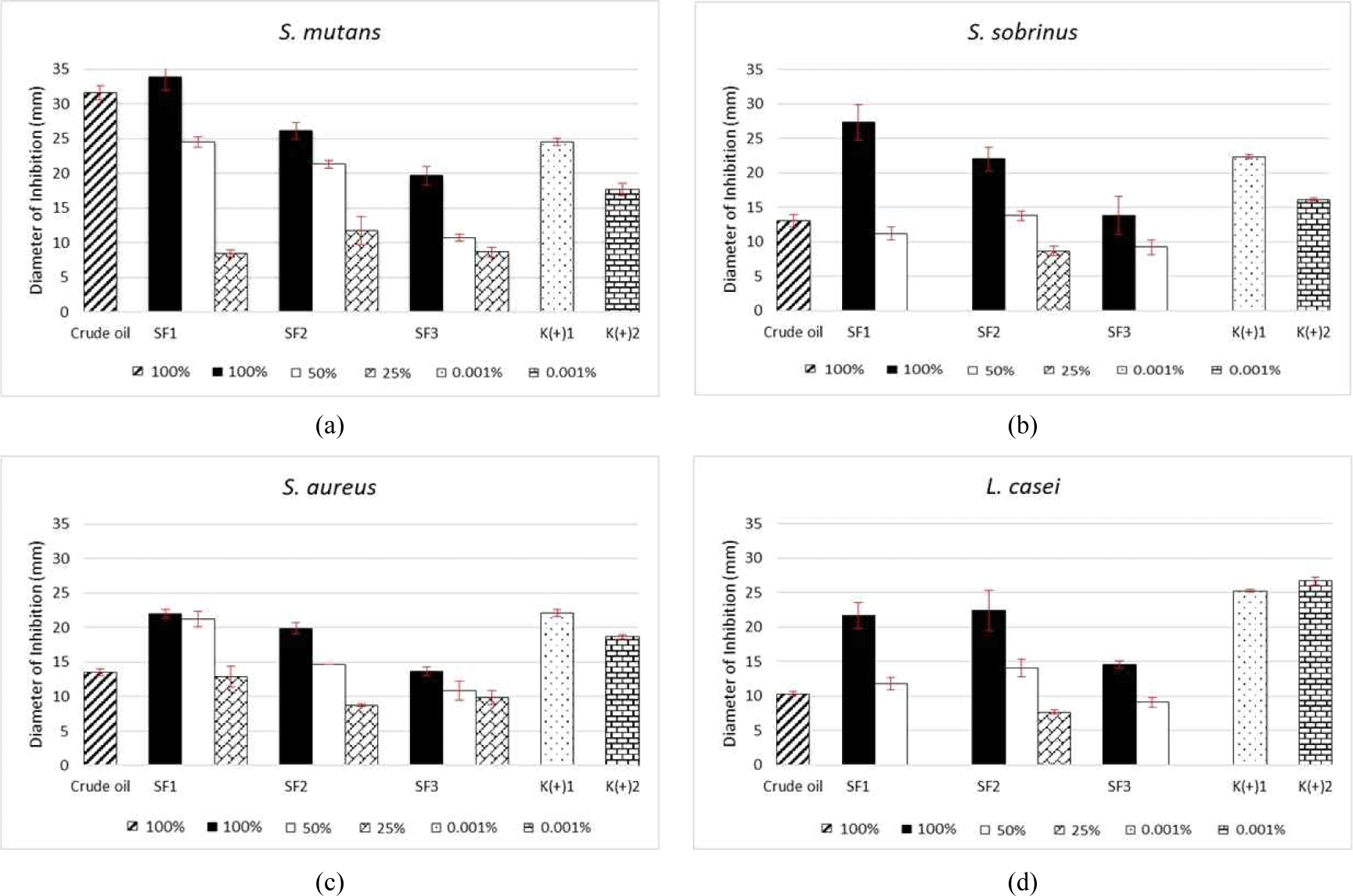
All fractions examined at the three concentrations highly inhibited S. mutans [Fig. 1(a)], with decreasing effectiveness as the fraction concentration was decreased (100% > 50% > 25%). Each pure fraction inhibited the growth of S. mutans, with the highest inhibition value observed for the SF1 fraction (33.89 mm), followed by SF2 (26.11 mm) and SF3 (19.67 mm). These results indicate that at a concentration of 50%, the samples inhibited bacteria compared to the same extent as both positive controls, with a value of 24.56 mm for the SF1 fraction. This inhibition value was equal to that of chloramphenicol. High inhibition was also observed for the 50% SF2 fraction (21.33 mm), whereas at a concentration of 25%, the inhibition ability of the three fractions did not surpass the activity of the standards. The high inhibition value of the SF1 fraction was likely related to the dominant compound in the fraction, α-phellandrene (47.97%), as well as other compounds in the monoterpene group. Işcan et al. (2012) reported that α-phellandrene exerts antimicrobial, antibacterial, and fungicidal activities. Subsequently, İşcan (2017) found that α-phellandrene of the hydrocarbon monoterpene group inhibited Enterobacter aerogenes, Serratia marcescens, E. coli, S. typhimurium, B. cereus, S. aureus, Listeria monocytogenes, and Candida spp. Ghasemi Pirbalouti and Gholipour (2016) and Buitrago et al. (2012) found that an essential oil with antibacterial activity contained > 30% α-phellandrene as the main compound. According to Zhang et al. (2017), the mycelia growth of P. cyclopium was significantly inhibited by α-phellandrene and nonanal by severely disrupting the integrity of the fungal cell membrane, resulting in leakage of cell constituents and potassium ions. Additionally, these substances trigger an increase in total lipid content, extracellular pH, and membrane permeability.
The largest inhibition value (27.33 mm) in the antibacterial test against S. sobrinus was observed for the SF1 fraction; however, inhibition drastically decreased at a concentration of 50%, with no inhibition at 25% (Fig. 1). Fraction SF2 showed the second-largest inhibition value, with an inhibition diameter of 22.00 mm, which was higher than that of the chlorhexidine positive control. Similar to SF1, 25% SF3 exhibited no activity against the tested bacteria. In addition to the dominant α-phellandrene, the SF1 fraction contained p-cymene in monoterpene group as a main compound. p-Cymene is the main antimicrobial compound found in thyme. Considerable evidence suggests that monoterpenes have antibacterial, antivirus, and antifungal activities. Furthermore, p-cymene inhibits tumor proliferation through antiangiogenic mechanisms, cytotoxicity against cancer cells, and anti-adhesion action (Kaluđerović et al., 2015; Li et al., 2016; Păunescu et al., 2015). Recent observations indicate that p-cymene decreases biofilm development by Burkholderia xenovorans by accumulating in bacterial membranes and altering the membrane structure (Agulló et al., 2017). Additional studies indicated that hydrocarbons and oxygenated monoterpenes possess a wide spectrum of antimicrobial activities against pathogenic microorganisms (Badawy et al., 2019; Garcia et al., 2008; Marei et al., 2019; Niksic et al., 2017).
Analysis of S. aureus, showed that the SF1 pure fraction was the most active inhibitor, with an inhibitor diameter of 22.00 mm; this fraction was also active at a concentration of 50% [21.22 mm; Fig. 1(c)]. This result indicates that this diluted fraction also has inhibition effects. For SF2, the inhibition zone was larger than that of chlorhexidine, which was 19.89 mm (pure fraction). All concentrations applied to S. aureus inhibited the bacteria by up to 25%. SF1 showed the second largest inhibition zone at a 50% concentration, followed by SF2 with an inhibition value of 14.67 mm, whereas the smallest was observed for SF3 (10.89 mm). At a concentration of 25%, SF1 achieved the largest inhibition value of 12.89 mm, whereas the lowest value was observed for SF2 (8.78 mm). These results indicate that SF1 had the strongest activity at a concentration of 25% (12.89–22.00 mm). In North Kalimantan, L. angulata plants are traditionally used to cure blains, a skin infection commonly caused by S. aureus (Ariyani and Mustikasari, 2010; McCaig et al., 2006). Kuspradini et al. (2019) showed that the leaves, bark, and branches of L. angulata inhibited S. aureus with a KHM value of 156.25 ppm, which was stronger than the effects of the positive control (100 ppm). Other members of the monoterpene group, such as citral, citral epoxide, and linalool, also exhibit high activity against methicillin-resistant S. aureus (Saddiq and Khayyat, 2010; Silva et al., 2015).
Based on the results for L. casei [Fig. 1(d)], the highest inhibition was observed for SF2 (pure), followed by SF1 and SF3. These fractions showed inhibitory activity at a 50% concentration, with the largest diameter (14.11 mm) in SF2 and smallest (9.11 mm) in SF3. SF1 and SF3 at concentrations of 25% showed no inhibitory effects. As the main compounds, SF2 contained α-phellandrene and trans-caryophyllene, which are members of the monoterpene and sesquiterpene groups, respectively. Trans-caryophyllene exhibits bactericidal effects against pathogenic oral bacteria (Azizan et al., 2017). Sesquiterpenes act as antibacterial agents by compromising the permeability of bacterial cell membranes, thereby inhibiting bacterial growth (Hapsari and Feroniasanti, 2019). Monoterpenes and sesquiterpenes are the most common compounds found in essential oils and have a broad spectrum of pharmacological properties, including antimicrobial activity (Bayala et al., 2014; De Cássia da Silveira e Sá et al., 2013; Geroushi et al., 2011).
Analysis of the antibacterial effects of the oil fractions of L. angulata oil, which were collected using SB distillation as shown in Fig. 2, revealed activities against the four test bacteria. Similar to the activities observed following SF distillation, SB6 showed the largest inhibition value; this fraction was rich in α-phellandrene (21.44%), with a diameter of 35.78 mm, surpassing the effects of both positive controls. At the 50% concentration, the samples inhibited bacteria to a greater extent than both positive controls, with SB6 showing a value of 29.67 mm. Interestingly, the inhibition value of SB7 at a 25% concentration still outperformed chlorhexidine, exhibiting a value of 20.83 mm, which was the largest value at this concentration level. High antibacterial activity was observed for fractions abundant in sesquiterpene compounds, such as SB7, as these compounds effectively inhibit several microorganisms such as gram-negative and gram-positive, as well as some fungal species including B. subtilis, S. aureus, E. coli, Pseudomonas aeruginosa, Proteus sp., Enterococcus faecalis, oral bacteria (aerobic S. mutans, S. mitis, S. sanguinis, and S. sobrinus and anaerobic Prevotella nigrescens, Porphyromonas gingivalis, Actinomyces naeslundii, and Bacteroides fragilis; Bach et al., 2011; Barrero et al., 2005; Diastuti et al., 2014; Sousa et al., 2015). A study conducted by Kuspradini et al. (2019) on the extraction of n-hexane, ethyl acetate, and ethanol from L. angulata revealed minimum inhibitory concentrations of 156.25 ppm for inhibition of S. mutans growth.
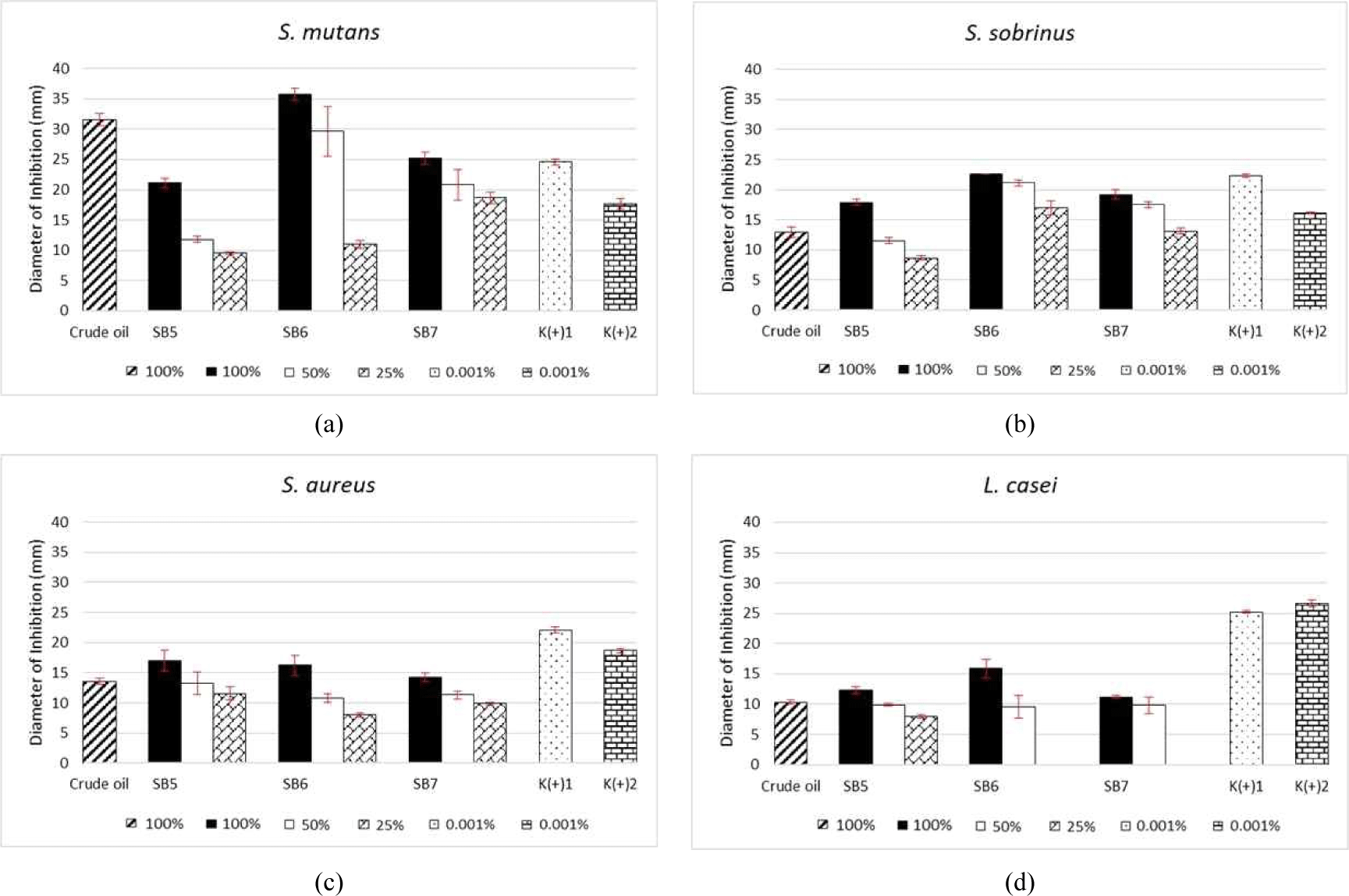
In analysis of the antibacterial activity against S. sobrinus, the largest diameter was observed for fraction SB6 (22.67 mm), followed by fractions SB7 (19.22 mm) and SB5 [17.89 mm; Fig. 2(a)]. Only the pure SB6 fraction surpassed the inhibition ability of both control antibiotics, whereas the other two pure fractions showed higher effects than chlorhexidine. All tested samples showed activity at a 50% concentration; the diameters from highest to lowest were SB6 > SB7 > SB5. These fractions, produced by SB distillation, inhibited S. sobrinus at up to 25%, with fraction SB5 achieving the smallest inhibition value (8.67 mm). Fraction SB6 was rich in chemical components of the monoterpene group, which strongly inhibits S. sobrinus. Alipour et al. (2015) reported that the strong antibacterial effect of essential oil from Ferula cupularis flowers, leaves, and stems was caused by the high content of hydrocarbon monoterpenes in such oils. Previous studies (El-Shenawy et al., 2015; Kotan et al., 2007; Zhu et al., 2013) showed that an essential oil with a high oxygenated monoterpene content was a strong antimicrobial agent.
As shown in Fig. 2(c), the lowest-concentration fractions of L. angulata (25%) generated using SB distillation actively inhibited S. aureus. The largest inhibition value was observed for fraction SB5 (17.00 mm); however, none of the fractions surpassed the effects of the positive controls. The second-largest value was observed for SB6, followed by SB7. The SB5 fraction contained some main compounds, namely α-phellandrene and p-cymene, from the monoterpene group, which are widely known for their pharmacological properties. Some monoterpenes, such as (+)-enantiomer, α-pinene, and β-pinene, exhibit antibacterial activity against C. albicans, Cryptococcus neoformans, Rhizopus oryzae, and methicillin-resistant S. aureus (da Silva et al., 2012). The essential oil from Litsea plant (i.e., L. cubeba) exhibits activity against S. aureus (Hu et al., 2019; Kačániová et al., 2020; Su and Ho, 2016; Thielmann et al., 2019; Van et al., 2016).
In examination of L. casei [Fig. 2(d)], the largest inhibition value was found in the SB6 fraction (15.89 mm), which remained active at 50% concentration. However, at a concentration of 25%, SB6 and SB7 showed no activity. The activity of the three fractions did not exceed that of the two control antibiotics. Essential oils, which contain members of the monoterpene group as the main compounds, exhibit antimicrobial activity against several strains of foodborne pathogens, both gram-positive and gram-negative, as well as against useful Lactobacilli strains (De Martino et al., 2009). In addition to containing α-phellandrene and p-cymene as the main compounds, fraction SB6 also contains ascaridole, which possesses anti-leishmanial (Monzote et al., 2018) and antimicrobial activities against phytopathogenic fungi and bacteria (Zefzoufi et al., 2020).
Thus, based on evaluation of the antibacterial activity from the pure fractions of L. angulata, fraction SF1, generated using the SF distillation method, showed the largest inhibition value, although the activities of fraction SB6 (collected from SB distillation) against S. mutans were insignificantly higher.
Based on evaluation of the antibacterial activity of the pure fractions of L. angulata, fraction SF1, prepared using SF distillation, had the largest inhibition value, although fraction SB6 (collected from SB distillation) showed slightly better activity against S. mutans. Fraction SF2 also had higher activity against L. casei, but the difference was less than 1 mm compared to that of SF1; thus, SF1 more effectively inhibited the four tested microorganisms, as it contained many hydrocarbon monoterpene compounds. The primary compound was α-phellandrene, which constituted over 40% of the sample. This compound exhibits various biological activities, including antibacterial, antifungal, and anticancer properties.
The antibacterial effects of different parts of L. angulata leaves towards the four tested bacteria more highly inhibited bacterial growth than the unseparated crude oil. Thus, separated essential oil is more effective than crude oil in inhibiting bacteria. Some studies indicated that separating an extract into fractions or single compounds leads to greater effects because the active compounds in plant extracts cannot synergize before separation by chromatography or other methods (Ahmad et al., 2011; Chua et al., 2019; Nguyen et al., 2017; Okwuchi, 2015; Onasanwo et al., 2008; Sannigrahi et al., 2010).
The size of the inhibition zone against microorganisms depends on the constituent chemical components in an essential oil, number of main components, concentration of the essential oil, and type and number of test bacteria (Baydar et al., 2004; Nazzaro et al., 2013). Essential oil disrupts the cell architecture first, causing damage to the membrane integrity and increasing permeability, which may interfere with numerous cellular activities. Essential oils can pass through the cell wall and cytoplasmic membrane and may disrupt or disturb different the fatty acid composition, phospholipid bilayer, and polysaccharide molecules. These changes may result in coagulation of the inner cell components of the cytoplasm and disrupt the bond between the fat and protein layer (Swamy et al., 2016). The release of cellular constituents in treated bacteria prompted the hypothesis that the primary effect of essential oils is membrane breakdown. Nonetheless, certain interactions with additional bacterial cell targets may strongly contribute to the observed antibacterial activities of the essential oil (Fadli et al., 2012). The structure of a compound is correlated with its microscopic characteristics, such as the molecular structure and macroscopic/empirical properties which include biological and molecular activities (Lee et al., 1996). Interactions between medicinal compounds and biological systems highly depend on the physical and chemical characteristics of the medicinal compounds, which are influenced by the types, amounts, and bonds between atoms, as well as the arrangement of the atomic space in the molecule (Utomo et al., 2017).
4. CONCLUSIONS
Essential oils from L. angulata leaves can be separated into fractions using both SF and SB distillation. The SF distillation method, particularly during the initial 60 min, yielded the highest oil content and produced fractions rich in bioactive compounds, especially α-phellandrene. GC-MS analysis confirmed that monoterpenes were the dominant constituents of the most active fractions.
Among all tested fractions, SF1 and SB6 exhibited the highest antibacterial activity, particularly against S. mutans, S. sobrinus, and S. aureus, whereas SF2 was the most effective against L. casei. Although antibacterial activity was observed at high concentrations (up to 100%), which were higher than the common minimum inhibitory concentrations (typically 0.01%–10%), the results reveal the application potential of these oils. Further research is needed to assess these activities at lower concentrations and to isolate specific bioactive compounds.
These findings highlight the potential of L. angulata essential oil, particularly its monoterpene-rich fractions, as a natural antibacterial agent for use in oral and dental products. Further in vivo investigations are needed to assess the safety, cytotoxicity, and efficacy of these fractions to support their application in pharmaceutical and personal care formulations.
