1. INTRODUCTION
Cajuput (Melaleuca cajuputi Powell) is a non-timber forest product of high economic value that is in great demand within the community. This tree species is native to Indonesia and widely cultivated for essential oil production (Juliarti et al., 2022; Putra et al., 2024). The leaves of the cajuput tree are the primary source of essential oil, which is obtained through distillation (Putri, 2023). In addition, cajuput offers additional benefits, including its use as firewood, construction materials, nectar sources, and honeybee pollen producers (Nguyen et al., 2019). The plant grows well in Indonesia, Thailand, and Vietnam (My et al., 2020) and demonstrates considerable potential in Java. Perum Perhutani manages 56,873.38 ha of cajuput plantations distributed across Central Java (12,018.88 ha), East Java (27,990.92 ha), West Java, and Banten (16,863.58 ha; Perhutani, 2023). In 2022, cajuput leaf distillation reached 47,622 t and cajuput oil production achieved 327 t, cumulatively generating 17.83 billion rupiahs (Perhutani, 2022).
In addition to its economic value, cajuput has important ecological benefits. This fast-growing species supports the restoration of critical terrestrial ecosystems. Previous research has shown that cajuput can thrive in wetland areas, provided that water depth remains within acceptable limits and the duration of waterlogging is not prolonged (Thanh et al., 2020). This suggests that cajuput may serve as a valuable species for restoring degraded peatlands, which are critical ecosystems providing important ecosystem services. Cajuput can also be used for contaminated soil remediation (Soboka and Yimer, 2022) and demonstrates high adaptability to various environmental conditions, including barren, acidic, and alkaline soils and even waterlogged areas (Hanif et al., 2018). Cajuput cultivation in agroforestry systems may contribute to food security programs (Suryanto et al., 2017).
However, Cajuput productivity is often affected by subterranean termite infestation. Puslitbang Perhutani and Faculty of Forestry Bogor Agricultural University (2019), reported large-scale subterranean termite attacks in various forest management units (KPH), including KPH Pasuruan (130.7 ha), KPH Saradan (117.5 ha), KPH Nganjuk (260 ha), KPH Pati (47.7 ha), KPH Semarang (40 ha), and several KPHs in West Java and Banten (700 ha). The total affected area reached 1,295.6 ha. Termites primarily damage plant roots and bark, disrupt nutrient distribution, and cause plants to wilt and die (Ngatiman and Fernandes, 2017). In KPH Kuningan and KPH Semarang, the frequencies of attacks were recorded at 14.85% and 15.39%, respectively, with heavy attack intensity causing mortality in 94.2%–97.09% of the affected plants (Puslitbang Perhutani and Faculty of Forestry Bogor Agricultural University, 2019).
Previous research has shown that cajuput roots contain bioactive compounds, including linoleic acid, which function as attractants and feeding stimulants for subterranean termites (Arinana et al., 2024). However, this research only used an n-hexane solvent, thereby limiting the identification of potentially more polar compounds. Paknahad et al. (2019) stated that solvent selection should align with the polarity of the target compound, with polar compounds dissolving in polar solvents and nonpolar compounds dissolving in nonpolar solvents.
Therefore, further research is required to identify the chemical compounds responsible for subterranean termite attacks on cajuput roots. This study used a multilevel maceration method using four solvents: n-hexane, ethyl acetate, methanol, and water. These solvents were chosen based on their varying polarities, facilitating the extraction of a broader range of compounds encompassing both nonpolar and polar substances (Septiana et al., 2023). The analysis was conducted using pyrolysis-gas chromatography mass spectrometry (Py-GCMS) and liquid chromatography mass spectrometry/mass spectrometry (LCMS/MS). Py-GCMS effectively analyzes macromolecules using gas chromatography, which breaks down compounds into volatile fragments (Picó and Barceló, 2020). Conversely, LC-MS/MS excels in detecting polar and less volatile compounds using electrospray ionization (ESI) techniques (Harmita et al., 2019). Combining these two instruments and using different solvents of varying polarities (n-hexane, ethyl acetate, methanol, and water) is anticipated to identify a broad range of bioactive compounds in cajuput seedling roots. This approach aims to uncover the specific chemical profiles associated with termite attacks and provide more targeted and effective management solutions for subterranean termites.
2. MATERIALS and METHODS
This research was conducted from June to September 2024 at the Forest Products Chemistry Laboratory, Faculty of Forestry and Environment, IPB University; Research Advanced Laboratory, IPB University; and the Biomass and Biomaterials Laboratory National Research and Innovation Agency (BRIN). Termite testing was conducted at the Termite Rearing Unit Laboratory, Department of Forest Products, Faculty of Forestry and Environment, IPB University.
The materials used were cajuput seedling roots (Eucalyptus sp.), Whatman No. 1 filter paper, n-hexane, ethyl acetate, methanol, distilled water, sand, 5% dimethyl sulfoxide (DMSO), and Coptotermes curvignathus Holmgren. The tools used included a rotary evaporator, Wiley mill, 40–60 mesh sieve, Erlenmeyer oven, desiccator, analytical balance, plastic net, acrylic tubes, Py-GC/MS, and LC-MS/MS.
The cajuput seedlings used in this study were derived from clone 59 and approximately three months old. They originated from the KPH Perhutani in Indramayu, West Java Province, and were cultivated in polybags with a diameter of 7.5 cm and a height of 20 cm.
Cajuput seedling roots were dried at room temperature for 24 h, ground using a Wiley mill, and sieved through a 40–60 mesh sieve. The moisture content of the powder was calculated using the following formula:
Extraction was performed using a multistage maceration method. Root powder (550 g) was extracted successively with n-hexane, ethyl acetate, methanol, and distilled water (1:5 ratio) for 72 h, with solvent replacement every 24 h. The filtrate was filtered and concentrated using a rotary evaporator at 40°C. The following formula was used to calculate the yield:
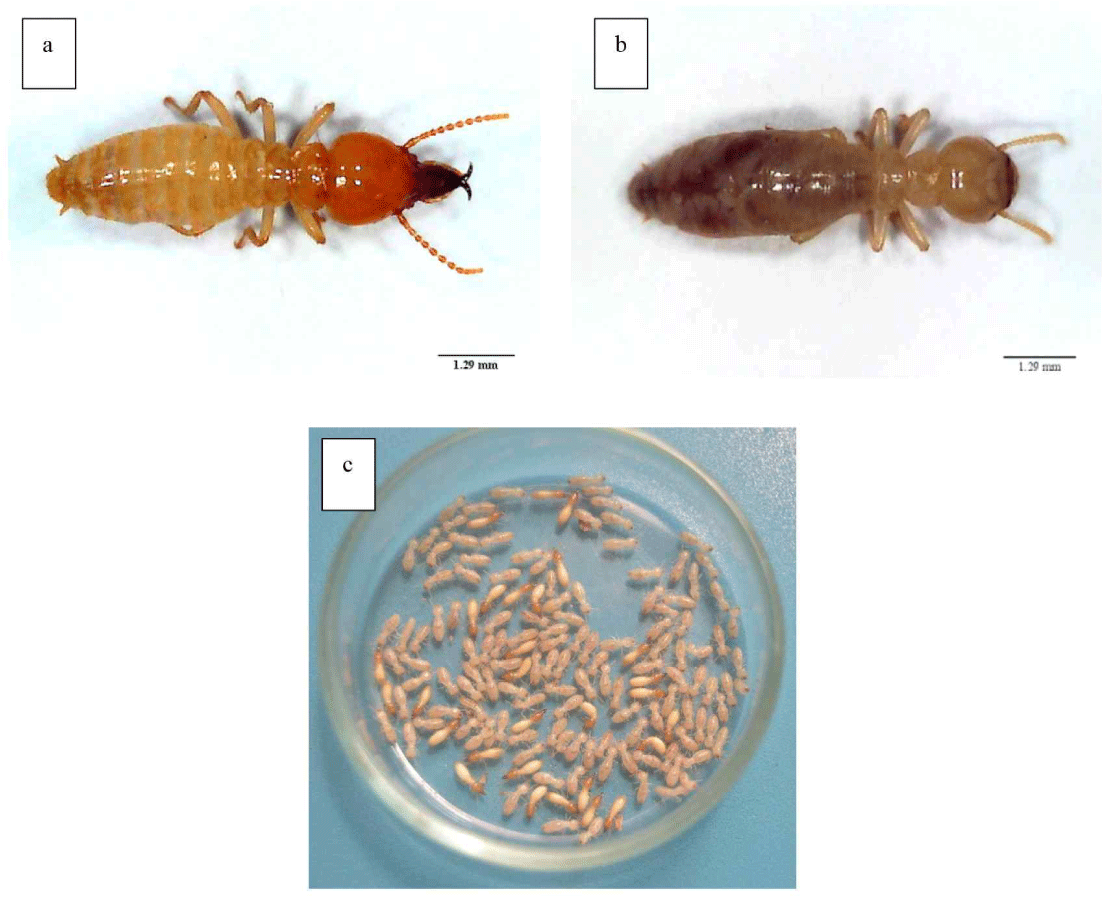
Bioactivity testing of the cajuput seedling root extract solution was conducted using the no-choice feeding bioassay method with hexaflumuron as the positive control. The no-choice feeding bioassay test procedure refers to JIS K1571:2010 as modified, using an acrylic tube (diameter, 8 cm; height, 6 cm; Fig. 1), the bottom of which was covered with dental cement (thickness, 1 cm). A 3 × 3 cm plastic net was placed at the center of the acrylic tube. The test container was then placed on a tissue sheet and moistened with distilled water. Termite bait in the form of a Whatman No. 1 filter paper (test paper) with a diameter of 50 mm was placed on a plastic net inside an acrylic tube. Next, 165 C. curvignathus specimens (Fig. 2), consisting of 150 workers and 15 soldiers, were placed in the acrylic tube.
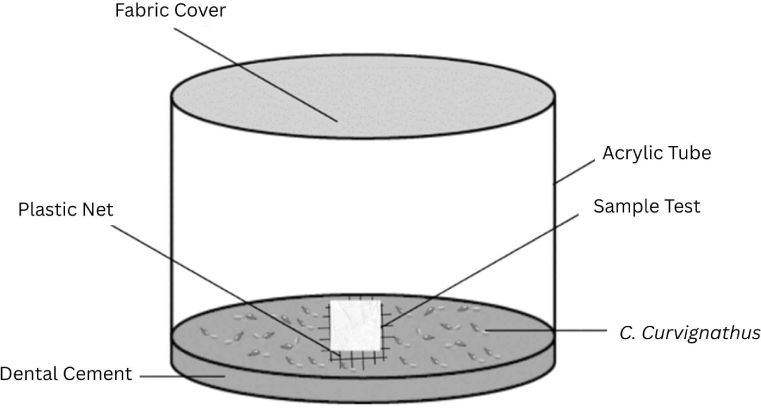
The filter paper was treated with the extract solutions by dispensing 550 μL onto its surface using a dropper pipette. After application, the filter paper was oven-dried at 60 ± 2°C for 48 h to remove residual solvent. After drying, the filter paper was weighed (W1) to record its initial weight. The test lasted 7 d, after which the container was disassembled, and the number of surviving C. curvignathus was counted. Each termite bait was cleaned and oven-dried at 60 ± 2°C for 48 h and then weighed (W2). The test mass loss was calculated using an equation based on JIS K 1571:2010:
P: mass loss, W1: paper dry weight before test (grams), W2: paper dry weight after test (grams).
The mortality of C. curvignathus was calculated using the following formula at the end of the test:
The median lethal concentration (LC50) of the cajuput seedling root extract solution was determined using probit analysis as described by Finney (1971). The database comprised the number of termite specimens that died at the end of the experiment (7 d of observation) at each solution concentration. The LC50 indicates the toxicity of the extract to termites and is classified into several classes (Table 1) based on Konan et al. (2022).
| LC50 (μg/mL) | Description |
|---|---|
| LC50 < 100 | Strong cytotoxic activity |
| 100 < LC50 < 500 | Moderate cytotoxic activity |
| 500 < LC50 < 1,000 | Low cytotoxic activity |
| LC50 > 1,000 | Non-toxic |
Data from Konan et al. (2022).
Phytochemical profile analysis using Py-GC/MS is detailed by Andika et al. (2023). A Shimadzu GC/MS system QP-2020 NX equipped with multishot pyrolysis was used. A total of approximately 5 mg of cajuput seedling root extract was placed in an SF PYI-EC50F eco-cup and covered with glass wool. The eco-cup was pyrolyzed at 500°C for 0.1 min using an SH-Rxi-5Sil MS column with a film thickness of 30 m × 0.25 mm × 0.25 μm with helium gas. The initial temperature was set at 50°C for 1 min, followed by an increase to 280°C at a heating rate of 5°C/min, and then maintaining 280°C for 13 min. The pressure was 20.0 kPa 15.9 mL/min, with a column flow of 0.61 mL/min and mass spectrum at 70 eV. The identification of pyrolysis products was conducted by comparing retention times and mass spectra using the National Institute of Standards and Technology (NIST) LIBRARY.
The second instrument used for phytochemical profile analysis was the ultra-high performance liquid chromatograph (UHPLC; Vanquish Tandem Q Exactive Plus Orbitrap HRMS; Thermo Fisher Scientific, Waltham, MA, USA), which refers to Familasari et al. (2023). The instrument had an AccucoreTM C18 column, 100 × 2.1 mm, 1.5 μm (Thermo Fisher Scientific). A total of 0.5 μL of filtrate was injected into the UHPLC-Q-Orbitrap-MS/MS (Thermo Fisher Scientific) after metabolites were separated using a gradient elution system for 50 min at a flow rate of 0.2 mL/min. The mobile phase composition used was 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), with a gradient elution system for 0–1 min (5% B), 1–25 min (5%–95% B), 25–28 min (95% B), 28–33 min (5% B). Positive ESI was used, with the Q-Orbitrap as the mass in ionization mode. The resolution used was 70,000 FWHM. The mass range was 100–1,500 m/z. The sheath flow rate was 15 mL/min, the auxiliary gas flow rate was 3 mL/min, the spray voltage (+) was 3.80 kV, the capillary temperature was 320°C, and the column temperature was 30°C. Complete MS/dd MS2 scanning was used. Mass spectra were obtained from UHPLC-Q-OrbitrapMS/MS and analyzed with Compound Discoverer version 2.3, MSDial, and MSFinder (Thermo Fisher Scientific). The databases used were mzcloud, ChemSpider, HMDB, and MassBank. The results of this instrument selected ten compounds with relatively high concentrations in each type of extract.
Literature studies were conducted on Google Scholar, PubChem, the NIST database, The Metabolomic Innovation Center (TMIC), and the Publish or Perish application with the keywords “attractant,” “subterranean termites,” “subterranean termite attractant,” and according to the 10 dominant compounds from each solvent and instrument as a subterranean termite attractant. The literature selected is under discussion: ten 10 dominant compounds as soil termite attractants.
Descriptive data analysis was performed using data generated from Py-GC/MS and LC-MS/MS, supplemented by literature studies from various sources, such as Scholar, PubMed, PubChem, NIST databases, and TMIC, to identify potential subterranean termite attractant compounds.
The variation in termite baits extracted with n-hexane, ethyl acetate, methanol, and distilled water at concentrations of 0.5%, 0.75%, and 1% was analyzed using IBM SPSS Statistics. Two-way analysis of variance (ANOVA) was used to analyze the weight loss and mortality of subterranean termites according to the concentration and solvent type. If the treatment demonstrated a significant effect, Duncan’s test was subsequently applied (Pallant, 2020).
3. RESULTS and DISCUSSION
The cajuput seedling root extract yield from Perum Perhutani KPH Indramayu was obtained through a multistage maceration method utilizing various solvents, namely n-hexane, ethyl acetate, methanol, and water. The yield was used to assess the effectiveness of the solvent during extraction. The yield ranged from 0.26%–2.08% (Fig. 3). The yield extract is affected by the ability of the solvent to dissolve a compound (Paknahad et al., 2019). The principle of the extraction process is similar to that of dissolution, in which the solvent dissolves compounds (extractive substances) with the same polarity (Nilamsari et al., 2023). The polarity of solvents is indicated by their dielectric constant values: water (81), methanol (33), ethyl acetate (6.02), and n-hexane (1.90), respectively (Adebayo et al., 2021; Galacia-Andrés et al., 2015; Kondrin et al., 2022). The higher the dielectric constant of a solvent, the higher its polarity. The principle of chemical extraction, known as the “like dissolves like” concept, indicates that polar compounds are soluble in polar solvents, whereas nonpolar compounds are soluble in nonpolar solvents (Paknahad et al., 2019). This principle is widely used to guide the selection of appropriate solvents for extracting and separating chemical compounds (Dobberpuhl et al., 2022). Polar solvents are effective in dissolving polar compounds, whereas nonpolar solvents are suited for dissolving nonpolar compounds. The extraction yields were determined based on the polarity of the solvent. According to Frankó et al. (2018), various factors, including temperature, duration, and particle size, are essential for influencing the outcomes of the extraction process.
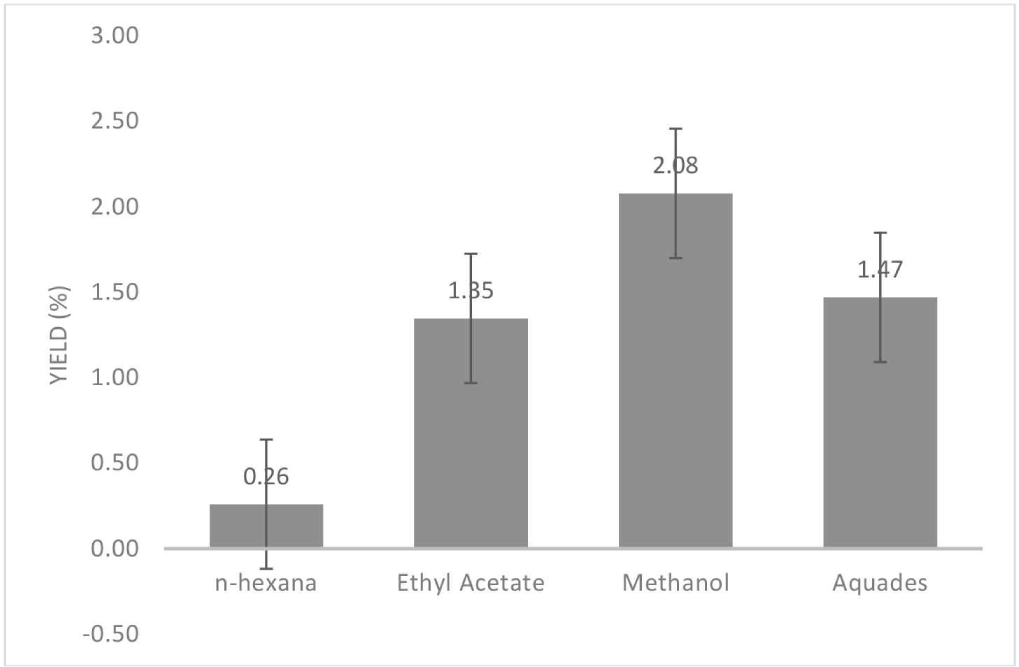
According to Hasnaeni et al. (2019), a high yield is related to the active compounds of a material or sample. The yield of the cajuput seedling root extract with n-hexane was the lowest. The finding is consistent with that reported in previous research, showing that nonpolar extracts obtained using n-hexane tend to have lower reactivity and conversion than polar extracts (Priscilla et al., 2017). This is because nonpolar n-hexane extracts primarily contain polycyclic aromatic compounds that are highly aromatic and less reactive (Priscilla et al., 2017). In contrast, the highest yield was obtained in the cajuput seedling root extract using methanol as the solvent. This aligns with the findings of Taşkin et al. (2021) for Scorzonera veratrifolia root extract, showing the highest polar compound content in a methanol extract.
The percentage yield is influenced by several factors, such as the extraction method, particle size, sample condition, storage time, extraction time length, sample-to-solvent ratio, and solvent type (Rosalinda et al., 2021). As a universal solvent, methanol can attract compounds with polar hydroxyl groups (–OH) and nonpolar carbon groups (–CH3). However, the dominance of polar groups in methanol increases its effectiveness for extracting specific polar compounds, such as phenolics, steroids, terpenoids, alkaloids, and glycosides (Indriatmoko et al., 2022).
Other solvents, such as distilled water, also form hydrogen bonds that increase the extraction efficiency of polar compounds, such as phenolics, alkaloids, flavonoids, tannins, and steroids (Nilamsari et al., 2023). Conversely, ethyl acetate, which is semi-polar, is less effective at attracting polar compounds because of its lower ability to form hydrogen bonds than methanol. The solvent n-hexane, which is nonpolar, can only dissolve lipids or oil compounds in the material, leading to the lowest yield (Hastuti et al., 2018; Hidayah et al., 2016).
The total yields of cajuput seedling root extracts obtained using different solvents were 0.26% for n-hexane, 1.35% for ethyl acetate, 2.08% for methanol, and 1.47% for water (Fig. 3). Extraction using all four solvents resulted in a total yield of 5.16%. Based on the classification of chemical components of broadleaf wood in Indonesia (Table 2), yields > 4% were categorized as high-grade extractive substances. This indicates the high potential of cajuput seedling roots as a source of bioactive compounds.
| Wood type | Composition class (%) | ||
|---|---|---|---|
| High | Moderate | Low | |
| Hardwood | 4 | 2–4 | 2 |
| Softwood | 7 | 5–7 | 5 |
Data from Jasni et al. (2016).
These results are relevant for developing wood-based products and environmentally friendly termite control materials. Further research is required to evaluate the effectiveness of bioactive compounds derived from cajuput seedling root extracts against particular termite species, such as C. curvignathus, recognized as a wood-destroying pest in Indonesia. Therefore, utilizing these bioactive compounds supports wood conservation and increases the value of cajuput root-based products.
The mass loss of the test paper was used to indicate the termite attraction to the extract applied to the filter paper. The mass loss of the filter paper was influenced by the solvent and concentration, with a maximum value of 26.96% in n-hexane solvent at a concentration of 1%. The ANOVA (α = 0.05) indicated that concentration and solvent affected mass loss (p < 0.05). This indicates the attraction of termites to the n-hexane test sample at a concentration of 1%.
Fig. 4 shows that the mass loss of the test paper varied considerably depending on the concentration and solvent added to the test paper. Variance analysis results (α = 0.05) showed that both solvent and concentration influenced the mass loss of the test paper (p < 0.05). The 1% n-hexane, exhibiting a value of 26.96%, was preferred and did not show a significant difference from the control (H) containing hexaflumuron. This indicates that the termites were similar to those of the test sample. A previous study by Arinana et al. (2024) on the extraction of cajuput seedling roots using n-hexane showed that linoleic acid compounds, which are feeding stimulants and exhibit bioactivity against insects, were present in this extract. Other dominant compounds in cajuput seedling root extract exhibit antibacterial, antimicrobial, and antifungal properties.
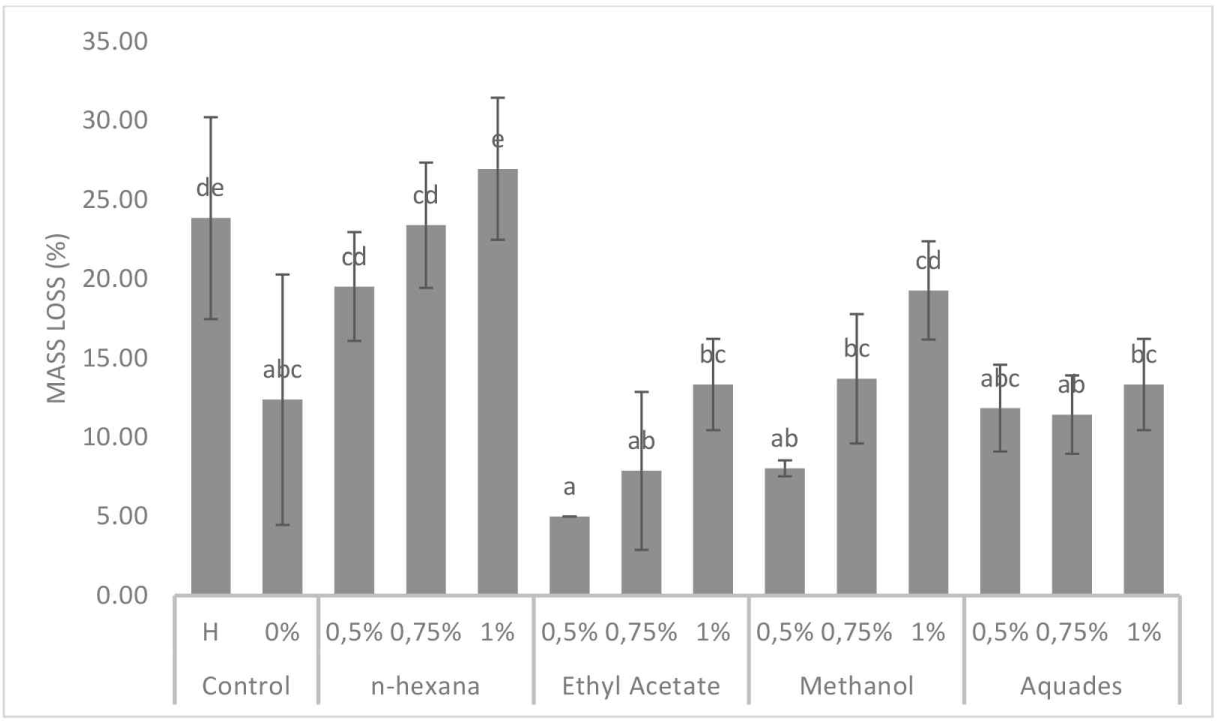
The pronounced effect of n-hexane on weight loss and termite mortality compared with that of other solvents can be attributed to its nonpolar nature, enabling effective extraction of nonpolar bioactive compounds (Rahayu et al., 2023). Compounds, such as linoleic acid, terpenoids, and phenolic derivatives, present in cajuput seedling root extracts exhibit high solubility in n-hexane. These compounds, particularly terpenoids, such as (E,E)-alpha-farnesene and eugenol, have been documented to function as termite attractants and feeding stimulants while simultaneously exhibiting toxic properties (Arinana et al., 2024). This dual functionality likely accounts for the higher mass loss and termite mortality observed in n-hexane-extracted samples. Additionally, the low polarity of the solvent enhances its ability to penetrate the termite cuticle and disrupt metabolic processes, thereby increasing its efficacy.
In line with these results, Carnohan et al. (2021) indicated that at a concentration of 1%, extract concentration affects termite feeding activity, with higher concentrations increasing termite preference for bait. The solvent type affects termite attraction, as each solvent possesses solubility properties that affect the dissolution of chemical compounds in cajuput seedling root extracts. n-hexane solvents, which are nonpolar and possess low polarity, can dissolve compounds with similar polarity and function as attractants (Fransiska et al., 2021). One of the compounds found in cajuput is terpenoids, such as mono-terpenes, α-pinene, and 1,8-cineole (also known as eucalyptol), which are known to have attractant properties (Boncan et al., 2020; Naidoo et al., 2018). The presence of (E,E)-alpha-farnesene, oleanolic acid, malinic acid, betaine, piperine, and nabilone in the phytochemical profile test may exert attractant and toxic effects on termites that affect mass loss.
Several researchers have reported on the efficacy of bioattractants against termites. Diba et al. (2022a) stated that basil leaves can act as termite attractants because they contain linalool, methyl chavikol, eugenol, methyl eugenol, flavonoids, and saponins, which can lure termites. Achmad et al. (2021) reported that pineapple peel extract may be a slow, toxic attractant for controlling termite attacks on rubber, oil palms, and several other types of plants in peatlands. Ganieva et al. (2023) showed that Hultemia persica flower extract is attractive to termites using the bioattractant bait system method. In addition, research by Plata-Rueda et al. (2018) showed that eucalyptus contains eugenol, caryophyllene oxide, α-pinene, α-humulene, and α-phellandrene compounds that are toxic and possess repellent effects on adult insects.
Phenol is thought to play a role in termite preference and mortality. The phenolic compound used in this study was nabilone, which is suspected to exhibit activity against insects, particularly termites. According to Indrayani et al. (2016), phenol is an aromatic compound found in plants, with a distinctive aroma that attracts termites. Phenols are chemical compounds commonly found in plants that function as termite attractants, drawing termites to food sources. In addition, phenols can help regulate the interactions between plants and microbes in the soil, potentially affecting the number of termites present. Arsyad et al. (2020) and Zalsabila et al. (2024) stated that the phenolic group is one type of extractive substance toxic to termites. Toxic compounds can be used to protect wood against termite attacks, which can substantially impact untreated wood (Hadi et al., 2018, 2020; Oramahi et al., 2022). Eugenol is a well-known phenolic compound (Kouznetsov and Méndez, 2022). According to Indrayani et al. (2016), eugenol has a dual effect; it is toxic to termites and can function as a termite bait.
In addition to phenolic compounds and their derivatives, eugenol and cajuput plants also contain terpenes. In this study, the terpenoid compound identified by LCMS/MS was (E,E)-alpha-farnesene. The (E,E)-α-farnesene synthase gene (GmAFS) is known for its role in producing insect-induced volatiles and aiding in nematode defense (Lin et al., 2016). It may also exhibit activity in attracting termites. Utilizing feeding activity to attract termites is recommended as a method to control termite activity via baiting. According to a previous study, extracts of Borneolum Syntheticum, Ephedra sinica, and menthol inhibited termite enzyme activity, and considerably decrease feeding behavior (Lee et al., 2020). Diba et al. (2017) stated that the active ingredient of termite bait must be nonrepellent and slow-acting to allow termite worker caste to transport the bait to the nest and distribute the active ingredient throughout the colony via trophallaxis. The condition of the filter paper is illustrated in Fig. 5.
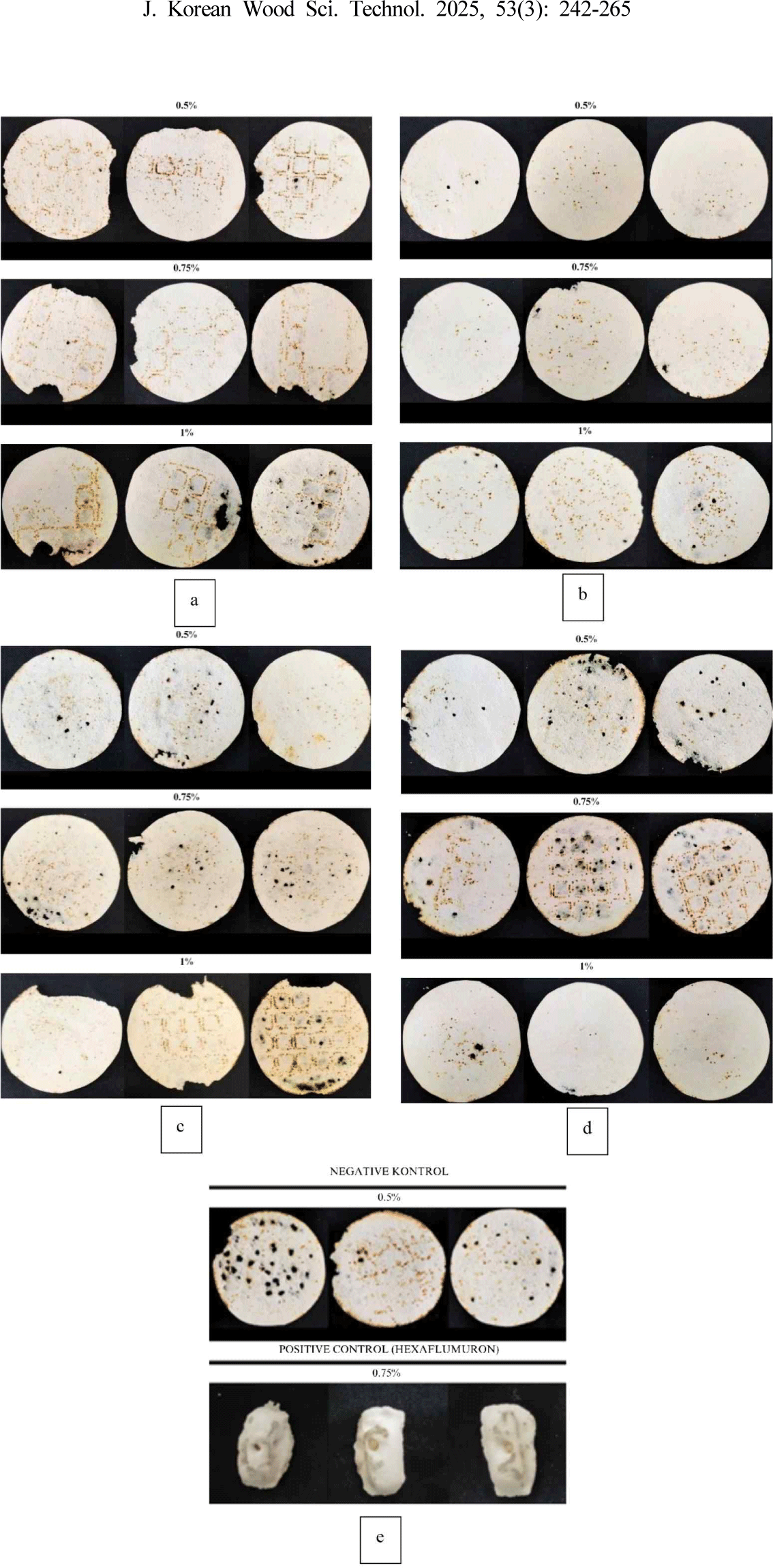
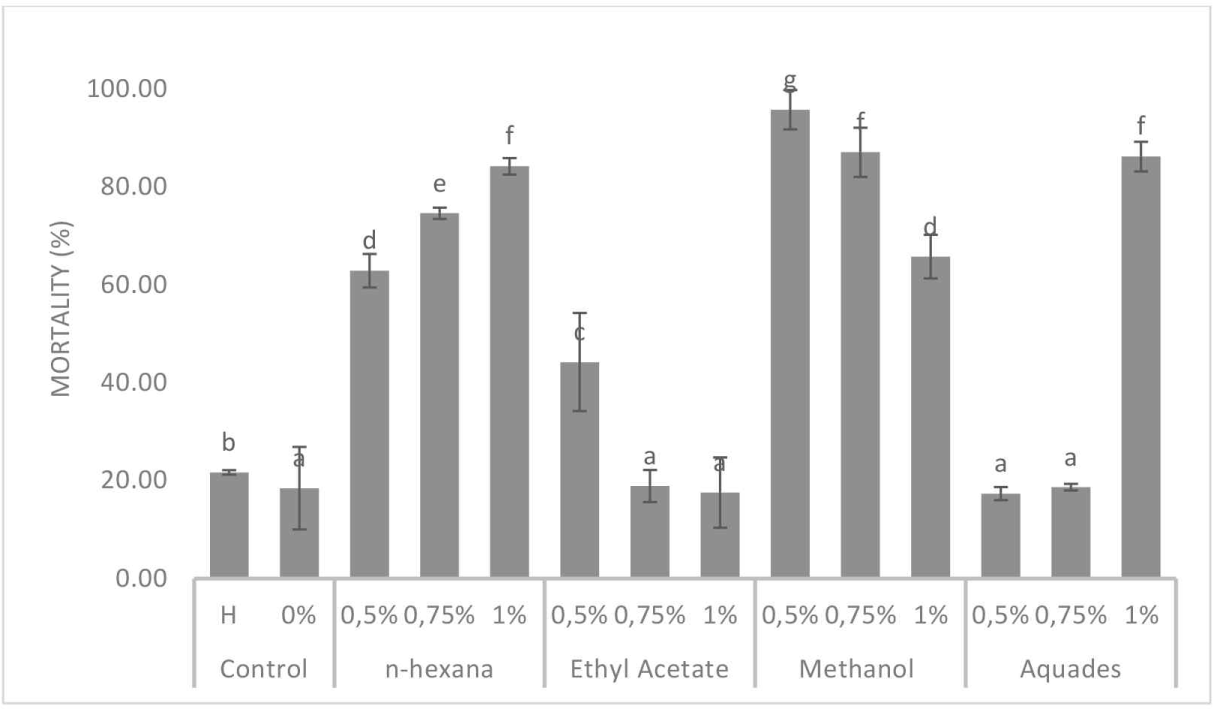
Termite mortality is the level of termite death that occurred during the 7-d test period. The results showed that the mortality of C. curvignathus termites varied from 17.33% to 95.78%, depending on the solvent and extract concentrations (Fig. 6). ANOVA (α = 0.05) indicated that the interaction of solvent and concentration affected termite mortality (p < 0.05). Duncan’s test showed significant differences, with the highest mortality observed with 0.5% methanol. This was followed by 1% distilled water, 1% n-hexane, and 0.75% methanol. The lowest mortality was observed in 0.5% distilled water, 1% ethyl acetate, 0% control, 0.75% distilled water, and 0.75% ethyl acetate, all of which were classified in the same group according to the Duncan’s test, with test values indicated by the letter “a.”
The results show that termite feeding activity is primarily localized within the same hole, resulting in minimal external alterations in the shape of the bait and more pronounced internal changes. According to Diba et al. (2022b), this is likely because termites create holes as burrows that allow them to come into direct contact with the bait. Mortality in the control group is attributed to the inability of termites to adapt and the scarcity of food sources (Chotikhun et al., 2024). Mirhaghparast et al. (2015) indicated that hexaflumuron, functioning as a chitin synthesis inhibitor, could eliminate termite colonies with slow action, no repellent effect, and dependence on the timing of application.
Fig. 6 shows the variation in termite mortality based on Duncan’s test. n-Hexane and distilled water showed an increase in mortality as the concentration increased. This is consistent with the findings of Alshehry et al. (2014) and Suhara (2020), which indicated that subterranean termite mortality in baits with low concentrations occurred more slowly because of the time required for the compounds in the bait to be absorbed by subterranean termites. In contrast, baits with higher concentrations exhibited a more rapid response and increased the mortality of subterranean termites. Conversely, while the ethyl acetate solvent at a concentration of 0.5% exhibited increasing mortality rates, the methanol solvent demonstrated an opposing trend, achieving the highest mortality rate of 95.78% at higher concentrations. Wibaldus and Ardiningsih (2016) observed an inverse relationship between the weight loss of the test paper and termite mortality. This nonlinearity is attributed to various factors, including environmental factors (e.g., high humidity), which can increase termite mortality and cannibalistic or necrophagic properties in termites (Nandika et al., 2015). Methanol solvent extract impacts termite mortality by delivering bioactive compounds that affect the termite gut and other critical physiological systems. The phenolic compound eugenol, identified in this study, is particularly toxic (Subekti and Saniaturrohmah, 2020).
The solvent type used affects the yield of dissolved compounds, subsequently affecting the resulting termite mortality. As stated by Kadir (2017), the compounds contained in the cajuput seedling root extract belong to the phenolic group that is toxic to termites. This study identified eugenol as one of the phenolic compound types. This finding was consistent with that of Lewis and Forschler (2016), who found that eugenol exhibits substantially greater toxicity to Coptotermes sp. than that observed in other chemical compounds, such as citral, citronellal, geraniol, and pyrrolidine. The effect of eugenol on termite mortality was associated with the bacteria present in the termite gut. Subekti and Saniaturrohmah (2020) stated that eugenol could change the permeability of bacterial cell membranes, leading to bacterial mortality. This affects termite mortality due to the loss of bacterial assistance in food degradation. Radek et al. (2023) reported that flagellate protozoa, including Pseudotrichonympha sp., Holomastigotoides sp., and Spirotrichonympha sp., inhabit the hindgut of termites. The mortality of protozoa owing to the consumption of bait containing bioactive compounds impairs the ability of termites to digest cellulose effectively, leading to insufficient energy acquisition and subsequent death.
The LC50 serves as a measure to assess the potency and toxicity of the extracts, with values based on the solvent used (Duffus, 1980; Kadir, 2017). The LC50 values of the solvents are listed in Table 3.
| Solvent | Regression equation | LC50 (μg/mL) |
|---|---|---|
| n-Hexane | y = 2.218x + 0.98 | 3,614.84 |
| Ethyl acetate | y = –27.176x – 1.0391 | 24,119 |
| Methanol | y = –43.149x + 0.4751 | 7,760.56 |
| Water | y = 6.324x + 0.6509 | 12,674.34 |
Table 3 shows that the n-hexane solvent exhibits the lowest LC50 value (3,614.84 μg/mL), indicating higher toxicity than the ethyl acetate solvent, which presents the highest LC50 value (24,119 μg/mL). Kadir (2017) stated that a lower LC50 value signified a more toxic material. Toxic phenolic compounds in cajuput plants contribute to their low LC50 values (Shanbhag and Sundararaj, 2013). The LC50 toxicity classification of cajuput seedling root indicated values > 1,000 μg/mL. According to Konan et al. (2022), LC50 values > 1,000 μg/mL indicate that the solvent has low toxicity.
This study indicates that n-hexane solvent can act as a poison and attractant for termite bait. This is owing to the lower LC50 toxicity of the n-hexane solvent extracts compared to that of other solvents, indicating its toxic potential. In addition, the highest weight loss value observed was 26.86% in 1% n-hexane, indicating substantial attraction of termites to the consumption of the test paper. The baiting system method results in the gradual mortality of termites following their consumption of test paper containing bioactive compounds. The worker caste executes this process is by transporting the bait to the nest and distributing the active compounds throughout the colony via the prophylactic method, thereby effectively eliminating termite colonies in an environmentally friendly manner. The formulation of the baiting system is an important factor in the success of termite baits because they must compete with alternative feeding sites (Iqbal et al., 2018).
The treatment outcomes using n-hexane extract of cajuput seedling roots indicated a weight loss of 26.86% in test paper at a concentration of 1%, with the lowest LC50 value recorded at 3,614.84 μg/mL. Based on these results, the n-hexane extract is identified as a potential bait for subterranean termites. Therefore, the n-hexane extract of cajuput seedling root may serve as an attractant for termites to encourage them to consume the bait.
The phytochemical analysis of cajuput seedling root extract was conducted using Py-GC/MS to determine the active compounds in the extract. Py-GC/MS analysis revealed that n-hexane, ethyl acetate, methanol, and water extracts contained 50, 50, 49, and 50 compounds, respectively.
Testing of the cajuput seedling root extract with four different solvent types revealed that not all solvents produced the same compounds (Table 4). Based on the test results listed in Table 4, n-hexane and ethyl acetate were identified as producing compounds similar in nature. Methanol and water solvents were identified to yield similar compounds. This is because of the distinct properties of these four solvents. n-Hexane is a straight-chain alkane hydrocarbon with six carbon atoms and exhibits minimal reactivity. It exhibits nonpolar properties for the extraction of nonpolar compounds (Rahayu et al., 2023). Ethyl acetate is a semi-polar solvent that attracts compounds with polar to nonpolar properties during extraction (Isliana et al., 2022). Water and methanol are solvents with polar properties but different polarities. Water is more polar than methanol (Galacia-Andrés et al., 2015; Kondrin et al., 2022). Therefore, n-hexane and ethyl acetate produced compounds that were identical to those in methanol and water solvents.
Each compound detected using Py-GC/MS exhibited different activities and did not necessarily have continuity between compounds. Acetic acid compounds were detected in each solvent at different concentrations (Table 4). Based on Heo et al. (2010), acetic acid is thought to affect termite mortality. The higher the concentration of acetic acid, the higher the termite mortality (Yatagai et al., 2002). Suprianto et al. (2023) indicated that the anti-mite activity of bark extract could be attributed to its chemical components, such as acetic acid, propanoate acid, phenols, and phenol derivatives. Acetic acid belongs to the organic acid compound category and is the only compound found in all four solvents.
In addition, several solvents produced the same compounds at varying concentrations. The compound 9-octadecenoic acid (Z)-, methyl ester was identified in three solvents: n-hexane, ethyl acetate, and methanol. However, this compound had the highest concentration (21.22%) in n-hexane soluble cajuput seedling root extract. Zhang et al. (2021) showed that the compound 9-octadecenoic acid (Z)-, methyl ester exhibited insecticidal properties, which had been proven effective in soap form. Its effectiveness was proven against larval stages of mosquitoes, such as Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus. The compounds 9-octadecenoic acid (Z)-, methyl ester, heptadecanoic acid, and methyl ester inhibited the fungal growth of all isolated phytopathogens to varying degrees of inhibition (Zaid et al., 2023).
The compound was subsequently identified in three solvents, namely n-hexane, ethyl acetate, and methanol. Hexadecanoic acid, methyl ester, also known as methyl palmitate, is an ester compound derived from the reaction between palmitic acid (a saturated fatty acid with 16 carbon atoms) and methanol. Hexadecanoic acid, methyl ester (methyl palmitate) is a fatty acid with antibacterial properties that damages the structure of the cell wall and membrane through a synergistic mechanism with various active compounds to increase the antibacterial activity (Padmini et al., 2010). In addition, hexadecanoic acid, methyl ester compound may contribute to the anti-termite activity of Azadirachtaexcelsa seed kernel extract (Adfa et al., 2023)
Methyl stearate compounds were identified in n-hexane-, ethyl acetate-, and methanol-soluble cajuput seedling root extracts. These compounds belong to the ester compound group. Among the three solvents, n-hexane exhibited the highest concentration of this compound at 4.71%. Lu et al. (2020) demonstrated that methyl stearate exhibited nematicidal effects against the root-knot nematode Meloidogyne incognita by reducing egg hatching and repelling juvenile nematodes. However, this study did not investigate their effects on insects. In general, methyl stearate is generally not classified as an attractant (substances that attract or tempt).
In contrast to the previous compounds, 2-methoxy-4-vinylphenol was identified in three solvents: ethyl acetate, methanol, and water. Flowers et al. (2022) suggested that 2-methoxy-4-vinylphenol was an aggregation pheromone produced by the red palm weevil, suggesting its potential role as a natural attractant for this insect pest.
Among all the compounds in the methanol-and ethyl-acetate-soluble cajuput seedling root extracts, some compounds have termite-attractant activity, namely eugenol. Eugenol can be reduced to several compounds, including methyl eugenol, which is a natural attractant (Kumar et al., 2024). This natural attractant is a pale yellowish-clear volatile liquid that has the same distinctive aroma as the original plant. The amount of eugenol used affected the active period of the attractant. The higher the content of this compound, the longer the active period as a termite attractant. Indrayani et al. (2016) tested crude extracts of clove (Syzygium aromaticum) and cajuput (Melaleuca leucadendra), which contain eugenol, against the subterranean termite Coptotermes formosanus. The extracts exhibited termiticidal activity. This proves that the termite-attractant properties in cajuput seedling roots can be determined through phytochemical testing using Py-GC/MS.
Phytochemical analysis of cajuput seedling root extracts was performed using LC-MS/MS to determine the content of active compounds in the cajuput seedling root. The highest relative concentration of each cajuput seedling root extract was observed for ethyl acetate. LC-MS/MS analysis showed that n-hexane, ethyl acetate, methanol, and water extracts contained 100 compound types.
Similar to the previous methods, LC-MS/MS produced diverse compounds in each solvent. However, this instrument identified compounds that were different from those of previous instruments (Table 5). The compounds identified using this instrument also exhibited different activities. Among all the compounds identified using this instrument, several were found to have bioactivity, including (-)-nabilone, (E,E)-alpha-farnesene, oleanolic acid, malinic acid, betaine, piperine, and nabilone.
As shown in Table 5, compound (-)-nabilone, which belongs to the phenolic compound group, was found in n-hexane-soluble cajuput seedling root extract at a concentration of 8.87% and in the ethyl-acetate-soluble extract at a concentration of 0.44%. Nabilone is a receptor agonist that has demonstrated potential therapeutic benefits in managing chronic non-cancer pain, neuropathic pain, PTSD-related nightmares, and insomnia (Cameron et al., 2014). Research indicates its ability to enhance non-rapid eye movement sleep and alleviate non-motor symptoms, such as anxiety and sleep disturbances in Parkinson’s disease (Peball et al., 2020). However, no specific research has been conducted on the activity of nabilones against insects, particularly termites.
Furthermore, the compound (E,E)-alpha-farnesene, belonging to the group of terpenoid compounds, was found in n-hexane-soluble cajuput seedling root extract at a concentration of 3.36% and in the ethyl-acetate-soluble extract at a concentration of 0.61%. Two potential roles for (E,E)-α-farnesene have been proposed: as a defense compound and as an attractant for animals that eat the ripe fruit and disperse the seeds (Pechous and Whitaker, 2004). Regarding the defense function, (E,E)-α-farnesene emitted due to Psylla infestation of pear trees is a potent attractant of predatory anthocorid insects (Pechous and Whitaker, 2004). Additionally, (E,E)-α-farnesene can subtly affect female codling moth behavior (Coracini et al., 2004). In addition to its roles in apples, (E,E)-α-farnesene has also been investigated in other plant species. In soybean, an (E,E)-α-farnesene synthase gene (GmAFS) was involved in the defense against nematodes and the synthesis of insect-induced volatiles (Lin et al., 2016). Owing to its activity, this compound has the potential to attract termites.
In addition, oleanolic acid compounds were found in n-hexane-soluble cajuput seedling root extract at a concentration of 2.42% and in the ethyl-acetate-soluble extract at a concentration of 1.26%. Oleanolic acid has demonstrated various pharmacological activities, including dose-dependent inhibitory effects against SARS-CoV-2 3CL protease (Kiriyama and Nochi, 2023) and HIV protease (Kaushik et al., 2021). It also exhibits anti-inflammatory, antioxidant, and antiviral properties against the dengue virus (Kim et al., 2015). Additionally, oleanolic acid shows antimicrobial activity against Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis, potentially by inhibiting peptidoglycan turnover and altering the muropeptide profile.
Subsequently, several compounds with bioactivity were also found, including maslinic acid compounds, which are included in the terpenoid compound group found in ethyl-acetate-soluble cajuput seedling root extract at a concentration of 1.79% and methanol-soluble at a concentration of 0.74%. Maslinic acid has been reported to possess a range of biological activities, including anti-inflammatory, antitumor, antidiabetic, and antioxidant effects (Wang et al., 2022). It exhibits anticancer properties against various cancers, such as gliomas (Pavel et al., 2019). It demonstrates anti-angiogenic activity by reducing in vitro capillary tube formation and suppressing vascular endothelial growth factor expression. In addition, betaine compounds were identified, which included a group of amine compounds found in the methanol-soluble cajuput seedling root extract at a concentration of 3.99% and water-soluble compounds at a concentration of 3.39%. Betaine compounds are organic compounds consisting of trimethylglycine, a derivative of the amino acid glycine, containing three methyl groups (-CH3) bound to nitrogen. This compound has bioactivity as an antibacterial (Chen et al., 2016). Despite their diverse pharmacological applications, there is limited evidence regarding the effects of maslinic acid and betaine on insects and termites.
Piperine is another compound thought to have bioactivity. This compound was included in the group of alkaloids found in the water-soluble cajuput seedling root extract at a concentration of 33.31%. This alkaloid is typically found in black pepper (Piper nigrum) and white pepper (Piper longum). This compound has anticancer, anti-inflammatory, and insecticidal properties (Li et al., 2022). While specific studies on their effects against insects and termites are limited, the diverse bioactivities of the compounds suggest potential activity against termites.
4. CONCLUSIONS
This study highlights the potential of cajuput seedling root extracts for sustainable termite management. n-Hexane extracts demonstrated strong attractant activity and considerable toxicity against subterranean termites, with an LC50 of 3,614.84 μg/mL and the highest mass loss (26.96% at 1% concentration). Phytochemical analyses revealed bioactive compounds, including (E,E)-α-farnesene and linoleic acid, supporting their application as termite attractants. These findings offer a sustainable alternative to synthetic chemicals that aligns with ecological conservation goals. Future studies should focus on isolating the active compounds, optimizing formulations, and assessing field applications to enhance their effectiveness.
