1. INTRODUCTION
Termites are insects that damage wooden buildings as well as wood cellulose derivatives in furniture, clothes, papers, and other items (Arinana et al., 2022; Lee et al., 2019, 2020; Subekti et al., 2018). The economic losses caused by termite attack in Indonesian housing reached IDR 8.7 trillion in 2015 (Arinana et al., 2019). From an economic perspective, the most destructive termite species in Indonesia are subterranean termite Coptotermes curvignathus Holmgren and drywood termite Cryptotermes cynocephalus Light (Subekti and Fadhila, 2023).
Termiticides are often used to control termite attack. These chemicals include imidacloprid, alphacypermethrin, aldrin, dieldrin, endrin, heptachlor, and mirex. Those pesticides including of persistent organic pollutants (POPs) are organic chemical substances, that is, they are carbon-based. They possess a particular combination of physical and chemical properties such that, once released into the environment, they: remain intact for exceptionally long periods of time (many years); become widely distributed throughout the environment as a result of natural processes involving soil, water and, most notably, air; accumulate in the living organisms including humans, and are found at higher concentrations at higher levels in the food chain; and are toxic to both humans and wildlife.
In addition to harming non-target insects and increasing resistance to target insect pests, synthetic termicides also present health concerns to humans (Pathak et al., 2022). Water contamination, biodiversity loss, bioaccumulation, and biomagnification will all result from these chemical residues (Ayilara et al., 2023). Using natural microorganisms, such as entomopathogenic fungi (EPF), for biocontrol purposes could prove to be the most efficient and environmentally sustainable way to control termites. Entomopathogenic fungal infection can occur through the digestive and respiratory systems, but especially through the outer surface layer (Altinok et al., 2019). In particular, EPF can kill termites by attaching to their surface and producing enzymes and toxins that enable sprout tubes to mechanically and chemically penetrate the integument. In addition, active fungal spores can be transmitted within termite colonies through trophallaxis (mutual feeding). EPF that have the potential to control insects include Metarhizium anisopliae, Beauveria bassiana, and Trichodermaharzianum.
Hussain et al. (2023) reported that M. anisopliae, B. bassiana, and T.harzianum were able to control fall armyworm Spodoptera frugiperda, with M. anisopliae being the most effective and mitigating 78.5% of the larval population. In a study by Calleri et al. (2010), M. anisopliae induced a mortality level of 83% in infected Incisitermes schwarzi (a species of drywood termites). Furthermore, Anggrawati and Ramadhania (2016) found that a conidial suspension of B. bassiana in MEB media led to an infection rate of 44.4% in termites, and Zhang et al. (2021) showed that Trichoderma sp. can effectively inhibit growth and reproduction of termites. In the latter study, that the mechanism of inhibition involved antibiosis and parasitism.
Termite control can be made more effective by combining toxic substances with attractants such as methyl eugenol that naturally occur in cellulose. The attract- and-kill principle is effective against pest species that live in cryptic habitats and complex environments that are hard to reach using simple techniques. This principle also makes use of trophallaxis in termite colonies (Achmad et al., 2021). Nanocellulose is useful as an inhibitor of termite growth activities and also as an antimicrobial. One benefit of nanocellulose is that the particles have a large surface-to-volume ratio, which makes attractants within the cellulose more detectable by termites. A liquid formulation containing nanocellulose and EPF could be used for termite control. The nanocellulose would serve as an attractant, while the EPF would work as a toxic substance to kill termites.
The innovation in the current study involves using nanotechnology to modify cellulose and combining the resultant nanocellulose with the three entomopathogenic fungal species to control termites. Our aim in this study was to analyze how effectively the combination of nanocellulose and the fungi M. anisopliae, B. bassiana, and T.harzianum is able to control subterranean termites and drywood termites. Parameters observed included weight loss from termite wood consumption (preference test), termite mortality percentage, and termite behavior test (like-dislike test). In addition, we analyzed the organic compounds contained in the EPF.
2. MATERIALS and METHODS
Two species of termites were used in this research. Subterranean termites and drywood termites were obtained from the colony collection in the Biology Laboratory of Universitas Negeri Semarang. The fungi for combination with nanocellulose, including M. anisopliae, B. bassiana, and T.harzianum, were obtained from the Integrated Laboratory for Bioproduct (iLab) National Research and Innovation Agency, Cibinong, West Java and the Microbiology Laboratory of Universitas Padjadjaran in West Java.
Fungi were propagated on solid media. Thirty-nine grams of potato dextrose agar (PDA) oxoid was mixed with 1 L of sterile distilled water. After homogeneity, the mixture was sterilized in an autoclave for 30 minutes at 121°C. Subsequently, 500 mg of chloramphenicol was added to the erlenmeyer flask, which was then sealed with aluminum foil and sterilized at 121°C for 15 minutes. Afterward, about 25 mL of media was poured into multiple petri dishes and allowed to solidify. Next, a cork borer was used to remove a plug of solid media from the plates containing fungal isolates, which was then planted in new solid media. The petri dishes were covered, wrapped, and taped tightly and then left at 28°C (room temperature) for 14 days for the fungi to grow (Azzahra et al., 2020).
The fungi that grew in the PDA were harvested and then suspended in 9 mL of sterile distilled water and vortexed for 1 minute to obtain the required spore density (dilution 10–7). Spore density was determined with a hemocytometer by dripping 0.2 mL on the calculation field and letting it stand for 1 minute. The number of conidia was calculated with × 400 magnification using the following formula:
Where S = spore density; R = average number of spores in five fields of view of the hemocytometer; P = dilution; and V = volume of the container (104) mL (Shackleton et al., 2015).
Liquid nanocellulose was produced by dissolving 20 g of microcrystalline cellulose in 2 L of distilled water, which was then put into a super grinder to create nanoparticles. Nanocellulose was used to make 20%, 40%, and 60% solutions by respectively mixing 20 mL, 40 mL, and 60 mL of the liquid nanocellulose in sterile distilled water up to 100 mL. After that, 60 mL of sodium tripolyphosphate solution (0.4%) was slowly added using a syringe. Next, 10 mL of EPF with area spore density was added, after which the sample was stirred at a speed of 300 rpm for 6 hours at room temperature. The solution was then incubated for 12 hours in the oven 45°C to destroy all microbes other than the fungi. The incubated sample was centrifuged at a speed of 2744 RCF for 20 minutes at 28°C. The supernatant was removed, while the pellet was cleansed with distilled water, and kept at –20°C for 2 days (Wu et al., 2021). The control used in this research was a 40% nanocellulose formulation. The composition of the control was the same as the 40% nanocellulose test formulation, namely there was a nanocellulose solution, sodium tripolyphosphate solution (0.4%), and 10 mL of EPF.
A mortality test (JIS, 2016) was used to determine the total number of drywood termites that died as a result of infection with EPF. Observations were carried out every day for 3 months.
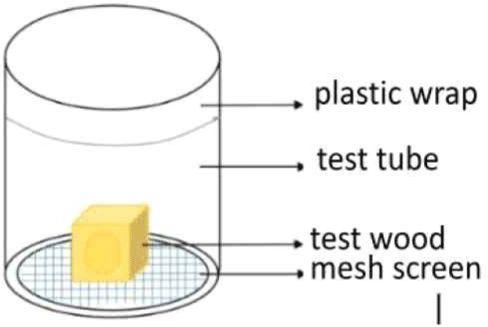
Wood pieces that were 2 cm × 2 cm × 2 cm in size were sprayed with 0.5 mL of a formulation and then dried and moved into a glass test container (Neves and Alves, 2000; Fig. 1). Thirty drywood termites were placed in each glass container, which was then covered with mesh screen and plastic wrap with holes, following Romano and Acda (2017).
The mortality test was conducted by calculating the total number of termites that died. Termites that were infected with EPF were removed so that the other termites were not contaminated by them. Observations were carried out every day for 3 months.
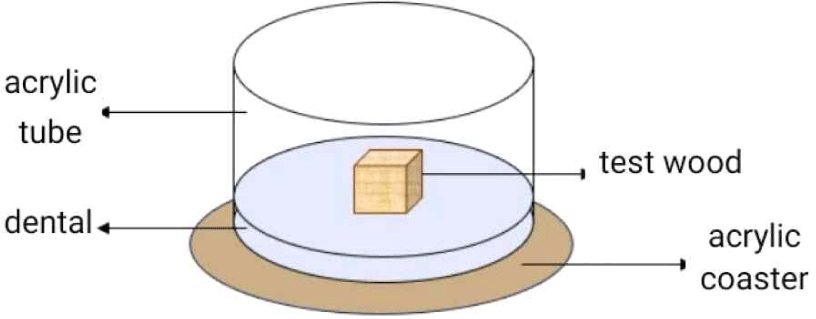
The mortality test (JIS, 2016) was carried out using cylindrical acrylic containers (8-cm diameter, 6-cm height; Fig. 2). The bottom of each container was covered with dental ± 1 cm thick. Dental are made from alginate, a powder-shaped impression material which, when mixed with water which is used as a research site so that the temperature and humidity match the termite habitat. Wood samples were sprayed with 0.5 mL of a formulation and then dried and moved into glass test (Neves and Alves, 2000). For control, the treatment was done by spraying 0.5 mL of 40% nanocellulose formulation. Fifty termites, including 43 workers and seven soldiers, were added, and all treatments were incubated for 30 days in a dark room (JIS, 2010). Observations were carried out every day for 30 days. The time of death was determined by how many days passed until death occurred (Indrayani and Fatmawati, 2019).
The field test of subterranean termites was based on a capture-mark-recapture method. At each station point (i.e., test site), a PVC pipe (10-cm diameter, 20-cm length) was placed at a depth of 25 cm in the ground in the vicinity of subterranean termite tunnels. All test woods were oven-dried at 60°C and weighed with an analytical balance before being inserted into a bait tool in a predetermined area. After 7 days, each station was observed for termite activity. The termites were fed with 1-mm-diameter Whatman paper that was sprayed with methylene red. The red color of termites was used to determine whether the termites had consumed the bait or not. Next, termites were counted and returned to the station based on the sampling. This method was repeated three times in the same colony (Su et al., 1991). Observation of termite mortality was done every 7 days, and the number of all dead termites in the bait tool was recorded. The amount of bait consumption in the sample test was calculated after the mortality reached 100%. The observations were conducted until termite mortality reached 100%.
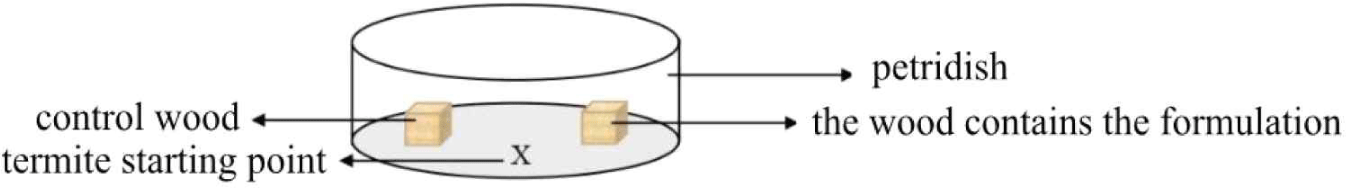
A bioassay (JIS, 2016) to determine termites’ behavioral response to the formulation was conducted in a petri dish (Fig. 3). Afterwards, the container was put in the tray containing distilled water. Wood samples that had been weighed were sprayed with 0.1 mL of the liquid nanocellulose formulation or the control solution and then placed in the petri dish. Inside the petri dish included two testing woods, which are control group (A) and another wood used nanocellulose formulations and enomopathogenic fungi (M. anisopliae, B. bassiana, and T.harzianum) respectively (B). Ten drywood termites OR subterranean termite were placed in the petri dish with the two wood samples. The observation in the petri dish was held every 10 minutes for 300 minutes to observe termites’ behavior (Oramahi and Yoshimura, 2013).
The analysis of organic material in EPF was carried out using gas chromatography-mass spectrometry (GC-MS) Shimadzu GC-2010 Plus series with a column temperature setting of 60°C held for 5 minutes, which was then raised 10°C per minute until it reached 250°C and held for 15 minutes. The injection temperature was 250°C, splitless injection mode. The MS ion source was set at 200°C and the interface was 250°C. The quantity of sample injected was 0.1 μL.
The preference test was calculated using a formula based on JIS (2010).
Where W1 = oven-dried sample before testing and W2 = oven-dried sample after testing.
The percentage of termite mortality was calculated using JIS (2010):
Where N1 = number of dead termites and N2 = total number of termites.
The percentage of mortality at each on-site observation was calculated using the formula of Kutana et al. (2018):
Where P1 = number of dead termites in all observations and P2 = number of dead termites in each observation.
Mortality tests and bait weight difference tests were statistically analyzed using one-way ANOVA to determine whether differences in the fungal species and the percentage of nanocellulose in the formulation had significant effects on mortality and termite preference levels. If an effect was found from the results of the analysis, it was further examined using the least significant difference (LSD) test. Behavioral tests (like-dislike) were statistically analyzed using t-tests.
3. RESULTS and DISCUSSION
The ANOVA results showed significant differences between treatments and the controls (p = 0.00 < 0.05), thus the different nanocellulose formulations and entomopathogenic fungushad an effect on the mortality of drywood termites (Table 1). To investigate the differences between treatment groups of nanocellulose formulation and entomopathogenic fungus fungi, further tests were conducted using the post hoc LSD test.
In Table 1 shows that in the control group, termite mortality was relatively low because the termites in this group received no treatment and storage conditions were conducive to survival. Based on the European standard EN118, an experiment is considered successful if two out of three samples in the control group have a survival value of > 50%. In this study, the control treatment group had low mortality and termite survival was > 50%.
M. anisopliae was the most effective type of fungus to control drywood termites because M. anisopliae has high toxicity that results in high termite mortality. In contrast, B. bassiana and T. harzianum induced relatively low mortality compared with M. anisopliae; these fungi are commonly used to inhibit the growth of several fungi that cause diseases in plants, including Rigidoporus lignosus, Fusarium oxysporum, Rhizoctonia solani, and Sclerotium rolfsii.
The EPF treatments with 60% nanocellulose were more effective because they induced 100% mortality in the fastest time, namely for 54 days. The results show that the higher the concentration of nanocellulose used, the higher the mortality rate of the termites. This outcome may have been due to termites not being able to easily recognize the volatiles released by fungi when nanocellulose concentrations were high, resulting in higher termite mortality (Hussain et al., 2010). This finding is supported by Popat et al. (2012), who reported that the use of nano-sized cellulose in pesticide formulations applied to insects can produce a vast area of infection (Table 2).
The biocontrol formulations showed similar results in both termite species. The higher termite mortality rate associated with the higher nanocellulose concentrations may be attributable to the high surface area containing fungal conidia. This will further increase the pathogenicity of the formulation despite termites being able to detect the presence of EPF. Baiting techniques by using fungi as the active ingredient have been widely used, both for soil insects and other household insect pests (Skinner et al., 2014). Sealing tunnel is often done to avoid the infection process of EPF (Staples and Milner, 2000).
The B. bassiana fungus works slowly and has a lower repellency level compared with the M. anisopliae strain. The T. harzianum fungus has no effectiveness under M. anisopliae and B. bassiana because T. harzianum fungus contains harzianum acid compounds. This compound has less effect on subterranean termites.
In the subterranean termite field test, termite activity was high at low concentrations (Nanocellulose 20%), and there was low variability in termite responses and delayed toxicity. However, field tests with B. bassiana showed better results than those with M. anisopliae and T. harzianum in reaching 100% mortality. This outcome could be due to termites exposed to B. bassiana fungus having a slower response that allowed them to survive longer and consequently spread the fungus more widely through tropalaxis.
Table 3 shows that the treatment with 60% nanocellulose formulation and M. anisopliae had an average mortality of 100% at the sixth week. In the field test, 100% mortality rate was reached on the 49th day. A long time was required to produce biological effects that could reduce food consumption level of termites and reach 100% mortality. EPF can reproduce and sustain virulence when growing in their habitat (Singh et al., 2011).
The attract-and-kill principle is effective against pest species with cryptic habitats and in complex environments that are usually inaccessible with typical application techniques. This principle utilizes the trophallaxis (mutual feeding) nature of termites in a colony (Achmad et al., 2021). Foraging termites will eat the bait and then spread the toxic active ingredient within the colony. Biocontrol formulations made with entomopathogenic fungus mixed with nano-sized cellulose is expected to be a powerful combination in termite control. The use of nano-sized cellulose functions to expand the active surface area of the biocontrol formulation, which can increase the pathogenicity of the active ingredients of the EPF (Popat et al., 2012).
The ANOVA results showed significant differences in the weight of wood samples treated with various formulations of entomopathogenic fungus and nanocellulose at p = 0.00 < 0.05. Thus, wood consumption by termites differed according to the specific formulations. To determine the differences between treatment groups, further tests were conducted using the post hoc LSD test. Data on the average weight loss of test wood were generated from the post hoc LSD test results.
Table 4 shows that the formulations with 60% nanocellulose and entomopathogenic fungus resulted in lower rates of termite food consumption and required a shorter time to produce biological effects on drywood termites. Termites consumed the test wood treated with entomopathogenic fungal spores because it contained nanocellulose as an attractant compound. However, the decrease in termite food consumption eventually led to death due to the inhibition of organelles and abnormalities in termites’ stomach function (Tables 5 and 6).
The T. harzianum fungus was associated with the least wood weight loss because termites continued to eat wood containing nanocellulose and T. harzianum has harzianum acid compounds that are slow acting. Harzianum acid has lower toxic properties compared with B. bassiana and M. anisopliae.
The results of the paired sample test in Table 7 show that the significance value in the like-dislike test of entomopathogenic fungus with nanocellulose had a degree of freedom value of 29; the significance value was p = 0.00 < 0.05. Thus, the hypothesis is accepted. The results of the like-dislike test on all treatments show that termites always chose the control group (nanocellulose only).
GC-MS results of ethylacetate extracts of all fungi showed that M. anisopliae, B. bassiana, and T. harzianum contained several secondary metabolites, with different contents of the main compounds between fungi. These compounds included palmitic acid, octadecenoic acid, 1,3 dipalmitin, oleic acid/(Z)-9-octodecanoid acid, capric acid (decanoic acid), and eugenol. As reported previously, these compounds have potential as insecticides. Palmitic acid is a fatty acid that has been found in various studies to have insecticidal properties. It has been reported to affect insects like aphids, grasshoppers, caterpillars, and leaf suckers (Aker et al., 2023; Saravanan, 2022). Palmitic acid, as one the phytochemicals in plant extracts, has shown insecticidal activities and is found in significant quantities in certain extracts such as castor bean n-hexane extract that have been suggested as potential insecticides (Al-Harbi et al., 2021; Sotelo-Leyva et al., 2022).
Oleic acid/(Z)-9-octadecenoic acid is another fatty acid that is known for its insecticidal properties. It has been found effective in the form of insecticidal soap, and it has been particularly noted for its effectiveness against various larval stages of mosquitoes such as Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus (Zhang et al., 2021) The C18:X acids, especially oleic and linoleic acids, have been reported as being effective against insect pests (Dasenaki et al., 2022; Nirouman et al., 2016).
Capric acid has been used in botanical products for the control of mosquitoes to interrupt disease transmission and is known to cause mortality in mosquito populations (Maw and House, 2012). It has also been indicated as one of the safer options for insecticides that would not endanger nontarget species like fish (Hikal et al., 2017; Zhang et al., 2022).
Eugenol has demonstrated a wide range of biological activities, including its use as an insecticide. It is effective against a variety of pests such as termites, mosquitoes, beetles, wasps, hornets, and other insects. Eugenol acts by targeting the nervous system of insects and can be used as a contact insecticide, a fumigant, or a feeding deterrent agent. It has also shown properties that make it toxic and repellent to certain beetles and ticks, in addition to its fumigant properties (Fernandes et al., 2020; Fulton et al., 2014). These compounds, through different mechanisms, contribute to the overall insecticidal properties of the extract. The presence of these compounds in the extract suggests potential applications in pest control.
In addition to these compounds, there are several specific compounds found in each of the EPF, as shown in Table 8.
4. CONCLUSIONS
EPF have a potential role in controlling subterranean termites and drywood termites. In the current study, the main cause of mortality among subterranean termites and drywood termites was exposure to EPF, including M. anisopliae, T. harzianum, and B. bassiana. In addition, the higher the concentration of nanocellulose used in the formulation, the higher the termite mortality. The formulation with 60% nanocellulose and M. anisopliae provided was the best subterranean termite and drywood termite control. In the field testing, M. anisopliae was associated with greater termite control than B. bassiana and T. harzianum. The main components of the three EPF are palmitic acid, octadecenoic acid, 1,3 dipalmitoyl/ oleic acid/(Z)-9-octadecanoic acid, capric acid (decanoic acid), and eugenol. The results of this research can be used for pest control management for subterranean termites and drywood termites.