1. INTRODUCTION
Bacterial infections pose a significant threat to human health, and the rise of drug-resistant bacteria has made the treatment of these infections increasingly difficult (Doron and Gorbach, 2008; Martínez and Baquero, 2002). One of the major challenges in the treatment of bacterial infections is the occurrence of persister cells, a small subpopulation of bacteria highly tolerant to antibiotics and other antimicrobial agents (Balaban et al., 2019). Similar to dormant cells, persister cells exhibit reduced metabolism, rendering antibiotics that target their metabolism ineffective (Lewis, 2007). Upon completion of antibiotic treatment, these cells can revert back to their normal antibiotic-sensitive states (Carvalho et al., 2017). The persister cell state enables bacteria to withstand antibiotic treatment without the emergence of resistant strains, making it a major contributor to chronic and recurrent infections and creating opportunities for resistant strains to emerge (Hansen et al., 2008; Wu et al., 2017). Persister cells can lead to prolonged hospital stays, increased healthcare costs, and heightened morbidity and mortality (LaFleur et al., 2010; Mulcahy et al., 2010). Therefore, the development of novel strategies targeting persister cells is crucial for the treatment of infectious diseases (Conlon, 2014). Several methods to reduce the number of persister cells have been reported, such as activating the ClpP protease with acyldepsipeptides (Lee et al., 2010; Li et al., 2010), boosting the proton-motive force with metabolites to enhance the uptake of aminoglycoside antibiotics (Allison et al., 2011), conjugating antibiotics with antimicrobial peptides (Brezden et al., 2016), and discovering new chemicals targeting cell membrane (Ooi et al., 2009).
Staphylococcus aureus, a pathogenic Gram-positive bacterium, is a significant cause of infectious diseases, including skin and soft tissue infections, as well as more serious systemic infections such as pneumonia, sepsis, and endocarditis (Oliveira et al., 2018; Otto, 2014). In a survey of bloodstream infections conducted in 45 countries from 1997 to 2016, S. aureus was the most frequently encountered strain, accounting for 20.7% of all cases (Diekema et al., 2019). S. aureus is also a common cause of persistent infections and poses a particular challenge, with numerous reports of antibiotic-resistant strains (Chang et al., 2015). Analysis of chronic S. aureus infection revealed that many cells were persister cells (Mulcahy et al., 2010).
Plants contain a wide variety of constituent compounds, many of which exhibit antibacterial (Ham et al., 2020), antifungal (Adfa et al., 2020; Jang et al., 2012; Lee et al., 2021; Yoon and Kim, 2023), anti-inflammatory (Myeong et al., 2023; Yang et al., 2019), and antioxidant effects (Lee et al., 2021; Nam et al., 2018; Yang et al., 2022). Coicis Semen, the seeds of the Coix lacryma-jobi plant, is a traditional medicinal herb commonly used in East Asian countries such as China, Japan, and Korea. Coicis Semen has been reported to possess anti-inflammatory cytokines (Yun et al., 2009), as well as antioxidant (Park and Kang, 2000) and anticancer (Das et al., 2017) properties. Additionally, several studies have suggested that Coicis Semen possesses bactericidal activity against a wide range of bacterial species, including S. aureus (Das et al., 2017). The main components of Coicis Semen oil are linoleic acid, oleic acid, and palmitic acid, constituting 38%–51%, 30%–38%, and 14%–18% of its total composition, respectively (Xi et al., 2016).
In this study, we evaluated the change in the number of persister S. aureus when an ethanol extract of Coicis Semen was co-administered with three antibiotics: oxacillin, ciprofloxacin, and tobramycin. Additionally, we explored the inhibitory effect of Coicis Semen extract components on persister cells and elucidated the biological mechanism of persister cell reduction.
2. MATERIALS and METHODS
S. aureus ATCC 6538 was acquired from the Korean Collection for Type Cultures (Jeongeup, Korea) and stored at –80°C with 25% glycerol (catalog number: 4066-4400, Daejung Chemical & Metals, Siheung, Korea). The cells were cultured using Tryptic Soy Broth (TSB, catalog number: 211825, Becton Dickinson Korea, Seoul, Korea), and Tryptic Soy Agar (TSA) was prepared by mixing TSB with 1.5% Bacto Agar (catalog number: 214010, Becton Dickinson Korea). For the cell cultures, the cells were cultured at 37°C with continuous shaking at 250 rpm. A saline solution was prepared by dissolving sodium chloride (catalog number: S0476, Samchun Chemicals, Seoul, Korea) at a concentration of 0.85% (w/v). All solutions were autoclaved at 121°C for 20 minutes.
Ciprofloxacin hydrochloride monohydrate (catalog number: C2227, Tokyo Chemical Industry, Tokyo, Japan), oxacillin sodium salt (catalog number: sc-224180B, Santa Cruz Biotechnology, Dallas, TX, USA), and tobramycin (catalog number: T2503, Tokyo Chemical Industry) were dissolved in distilled water prior to use. Linoleic acid (catalog number: L07949, Alfa Aesar, Thermo Fisher Scientific Korea, Seoul, Korea), oleic acid (catalog number: O0180, Tokyo Chemical Industry), and palmitic acid (catalog number: 129702500, Acros Organics, Thermo Fisher Scientific Korea) were dissolved in ethyl alcohol (catalog number: 000E1367, Samchun Chemicals) before use. Tween 80 (catalog number: 000T09) was obtained from Samchun Chemicals. SYTOXTM Green Nucleic Acid Stain (catalog number: S7020) was purchased from Invitrogen (Thermo Fisher Scientific Korea).
Coicis Semen was acquired from the Jiundang Oriental Pharmacy (Seoul, Korea), and its ethanol extract was prepared as described in a previous study (Ham and Kim, 2018) with slight modifications. Thirty grams of Coicis Semen were crushed to a diameter of ≤ 3 mm and soaked in 300 mL of 95% ethyl alcohol (catalog number: E0219, Samchun Chemicals) at 50°C for 3 hours, with periodic shaking every 30 minutes. The resulting extract solution was filtered using WhatmanTM qualitative filter paper Grade 1 (catalog number: 1002-110, CytivaTM, Sigma-Aldrich, St. Louis, MO, USA). The filtrate was then concentrated using a rotary concentrator (RV-10, IKA Korea, Seoul, Korea) and freeze-dried with a lyophilizer (FDU-2110, EYELA, SunilEyela, Seongnam, Korea) over the course of 3 days. The extract was stored at –80°C and reconstituted in ethanol before use.
S. aureus stored at –80°C was streaked on TSA plates and incubated at 37°C for 24 hours. A single colony was precultured in 5 mL TSB and incubated at 37°C with continuous shaking at 250 rpm for 24 hours. The precultured cells, with an Abs600 of 0.05, were inoculated in 5 mL of culture medium and incubated at 37°C with shaking at 250 rpm for 3 hours. Afterward, the cells were diluted in saline and spread on TSA plates. The plates were then incubated at 37°C for 24 hours to determine the initial colony count. Each antibiotic was added to the main culture medium at 10 times the minimal inhibition concentration (MIC): 2.5 mg/L for oxacillin, 20 mg/L for ciprofloxacin, and 80 mg/L for tobramycin. Ethanol was added as a control, and the extract or its chemical components were added to assess their impact on the persister cell population. After incubating at 37°C for 24 hours, the number of colony-forming units (CFUs) was examined on TSA plates as described in a previous study (Wang et al., 2018). The percentage of persister cells was calculated by dividing the number of CFUs after antibiotic treatment by the number of CFUs before antibiotic treatment. The experiment was conducted in triplicate. To evaluate the offsetting effect of the surfactant Tween 80, the change in the percentage of persister cells was measured with an additional treatment of 1% Tween 80 under the same conditions.
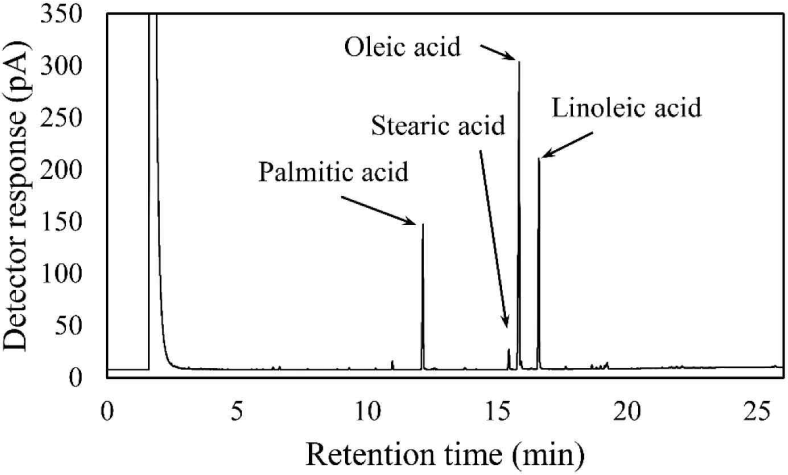
The components of the Coicis Semen ethanol extract were analyzed using gas chromatography as described in a previous study with slight modifications (Jandacek et al., 2004). The Coicis Semen ethanol extract (6.74 mg) was saponified with 4 mL of 0.5 N methanolic sodium hydroxide at 80°C for 5 minutes, followed by cooling at 25°C. The samples were then methylated with 3 mL of 14% boron trifluoride (catalog number: 021-06171, FUJIFILM Wako Pure Chemical, Osaka, Japan) at 80°C for 5 minutes, followed by cooling to 25°C. Next, the methylated samples were mixed with 2 mL of saturated sodium chloride solution and 2 mL of hexane. After vortexing for 1 minute, the hexane layer was separated by centrifugation at 455 × g and filtered with syringeless filters (catalog number: MV32ANPPT002TC01, GVS Korea, Namyangju, Korea). The fatty acid content of the Coicis Semen ethanol extracts was then analyzed via gas chromatography (model number: 7890B, Agilent Technologies, Wilmington, DE, USA) using a flame ionization detector with an HP-INNOWax column (catalog number: 19091N-133, Agilent Technologies). Helium was used as the carrier gas with a flow rate of 1 mL/ min. The inlet temperature was set at 250°C, and the detector temperature was also maintained at 250°C. The oven temperature was initially set at 140°C for 1 minute, then increased to 240°C at a rate of 3°C/min and held for 5 minutes. The overall run time was 26 minutes, with an injection volume of 1 μL analyzed in split mode (1:20).
Cell membrane permeability was assessed using SYTOXTM Green Nucleic Acid Stain according to the manufacturer’s instructions to examine the impact of Coicis Semen ethanol extract and its components (Kim et al., 2015). After an additional 3-hour incubation following the main culture, the S. aureus cells were washed twice with saline using centrifugation at 7,280 × g for 5 minutes. SYTOX™ Green Nucleic Acid Stain at a concentration of 0.5 μM was then incorporated into the cell suspensions, followed by the addition of the extract or its chemical components. Fluorescence was measured at 528 nm in a black 96-well plate (catalog number: 30296, SPL Life Sciences, Pocheon, Korea) using a Synergy LX multimode reader (BioTek, Agilent Technologies Korea, Seoul, Korea) with an excitation wavelength of 485 nm. The alteration in cell membrane permeability when treated with an additional 1% Tween 80 was also evaluated under the same conditions.
3. RESULTS and DISCUSSION
Several antibiotic-resistant strains of S. aureus have become increasingly notorious, with persister cells being among the main contributors to the emergence of resistant cells (Chang et al., 2020). Through a screening analysis using a library of methanol extracts from 389 forest products, Coicis Semen extract was found to substantially decrease the number of S. aureus persister cells in this study. During the screening, the number of persister cells for oxacillin was reduced by 95.5% using a 0.5 g/L methanol extract of Coicis Semen.
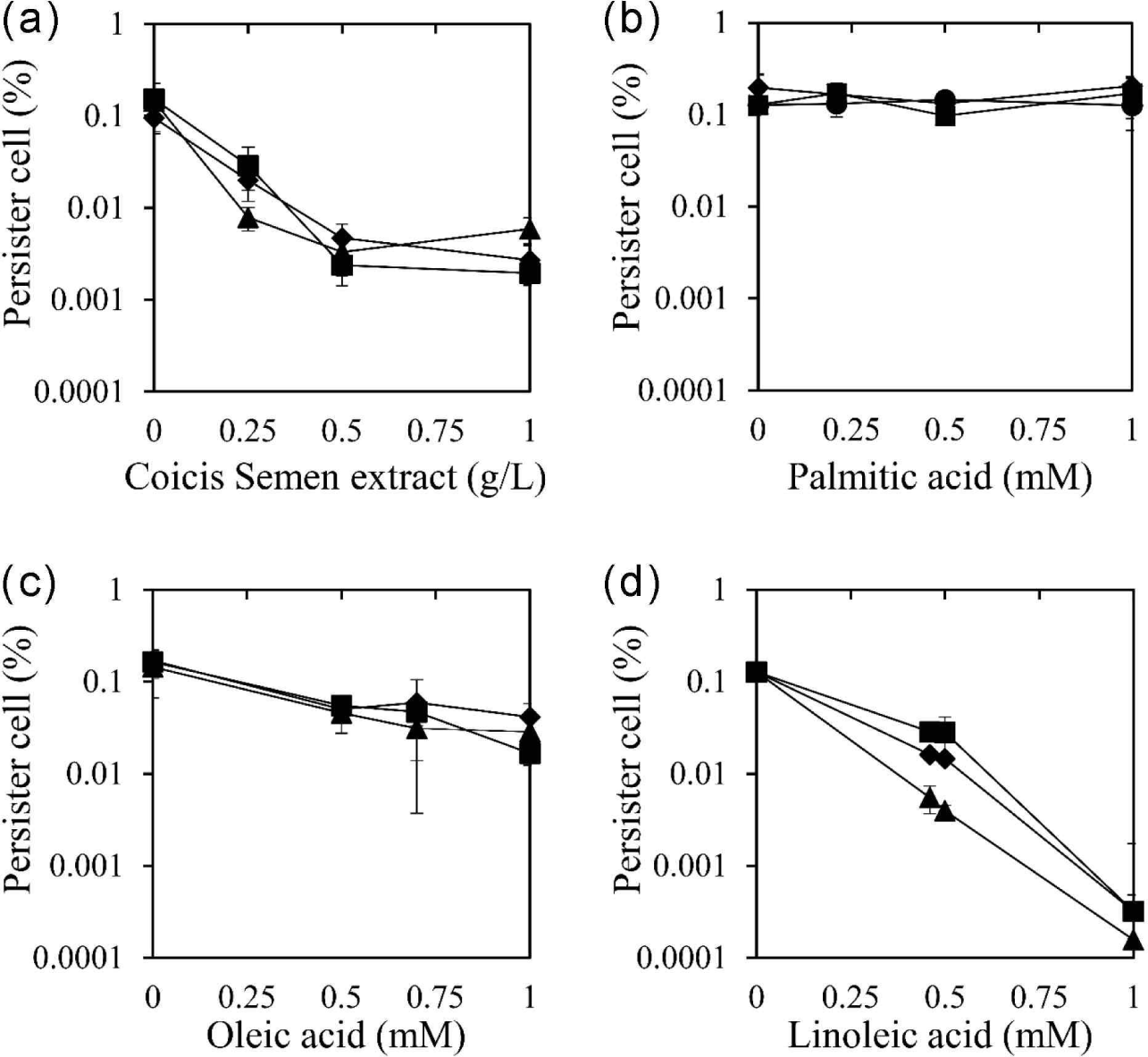
The identified Coicis Semen was further extracted with ethanol, a safe solvent, in a yield of 2.82%. The ethanolic extract of Coicis Semen was used to evaluate its persister cell reduction effect using three antibiotics: ciprofloxacin, oxacillin, and tobramycin [Fig. 1(a)]. These antibiotics were chosen due to their distinct bactericidal mechanisms. Ciprofloxacin inhibits DNA gyrase and topoisomerase IV, ultimately inhibiting cell division (Hooper et al., 1987). Oxacillin is a beta-lactam antibiotic that inhibits bacterial cell wall synthesis (Fernandes et al., 2013). Tobramycin binds to the bacterial ribosome and inhibits protein synthesis (Brötz-Oesterhelt and Brunner, 2008). The ethanol extract of Coicis Semen at 0.5 g/L reduced the number of persister cells by 98.4%, 95.1%, and 97.7% when co-administered with ciprofloxacin, oxacillin, and tobramycin, respectively. Moreover, the persister cell removal activity of the Coicis Semen ethanol extract became the maximum activity at 0.5 g/L when co-administered with ciprofloxacin, oxacillin, and tobramycin. The MIC of the ethanolic extract of Coicis Semen against S. aureus was above 1 g/L, and no antibiotic activity was observed below 1 g/L used in this study. These observations suggested that the reducing persister cells of the ethanol extract of Coicis Semen with antibiotics are not due to its antibacterial activity.
The fatty acid composition of Coicis Semen ethanol extract was analyzed using gas chromatography (Fig. 2). The fatty acid content consisted of 39.8% oleic acid, 26.0% linoleic acid, 11.0% palmitic acid, and 0.7% stearic acid. In a previous study, the free fatty acid content of an n-hexane extract of Coicis Semen was reported to consist of 45.8% oleic acid, 26.9% linoleic acid, 16.7% palmitic acid, and 5.5% stearic acid (Xi et al., 2016). Interestingly, despite the difference in the extraction solvent and analysis method in the aforementioned study, our free fatty acid analysis yielded a similar result.
To identify the active chemicals in the ethanol extract of Coicis Semen responsible for reducing persister cells, the effect was evaluated using the fatty acid components palmitic acid, oleic acid, and linoleic acid [Fig. 1(b–d)]. Oleic acid and linoleic acid demonstrated a reduction in persister cells for all three antibiotics tested, while palmitic acid did not exhibit this effect. When coupled with oxacillin, ciprofloxacin, and tobramycin, oleic acid at 0.5 mM reduced persister cells by 70.5%, 66.6%, and 68.2%, respectively [Fig. 1(c)]. Linoleic acid at 0.5 mM reduced persister cells by 88.5%, 78.0%, and 96.9% when co-administered with oxacillin, ciprofloxacin, and tobramycin, respectively [Fig. 1(d)]. Both oleic acid and linoleic acid displayed a concentration-dependent persister cell removal activity, with linoleic acid exhibiting a stronger effect than oleic acid. However, palmitic acid did not show a persister cell inhibitory effect [Fig. 1(b)]. Considering the content of palmitic acid, oleic acid, and linoleic acid in the Coicis Semen ethanol extract (0.21 mM palmitic acid, 0.7 mM oleic acid, 0.46 mM linoleic acid), the effectiveness of persister cell reduction at these concentrations was evaluated and depicted in Fig. 1. The assessment of the effect at these concentrations suggests that oleic acid and linoleic acid are the main active components in the Coicis Semen ethanol extract that reduce persister cells.
The reduction of persister cells by oleic acid and linoleic acid is likely related to their antimicrobial activity against S. aureus. In previous studies, the MIC of oleic acid and linoleic acid against S. aureus was reported as 0.9 mM (Atashbeyk et al., 2014; Fung et al., 2017) and 0.2 mM (Yuyama et al., 2020), respectively. This indicates that the antibacterial potency of linoleic acid is stronger than that of oleic acid, potentially explaining why linoleic acid reduced persister cells more effectively than oleic acid at the same concentration.
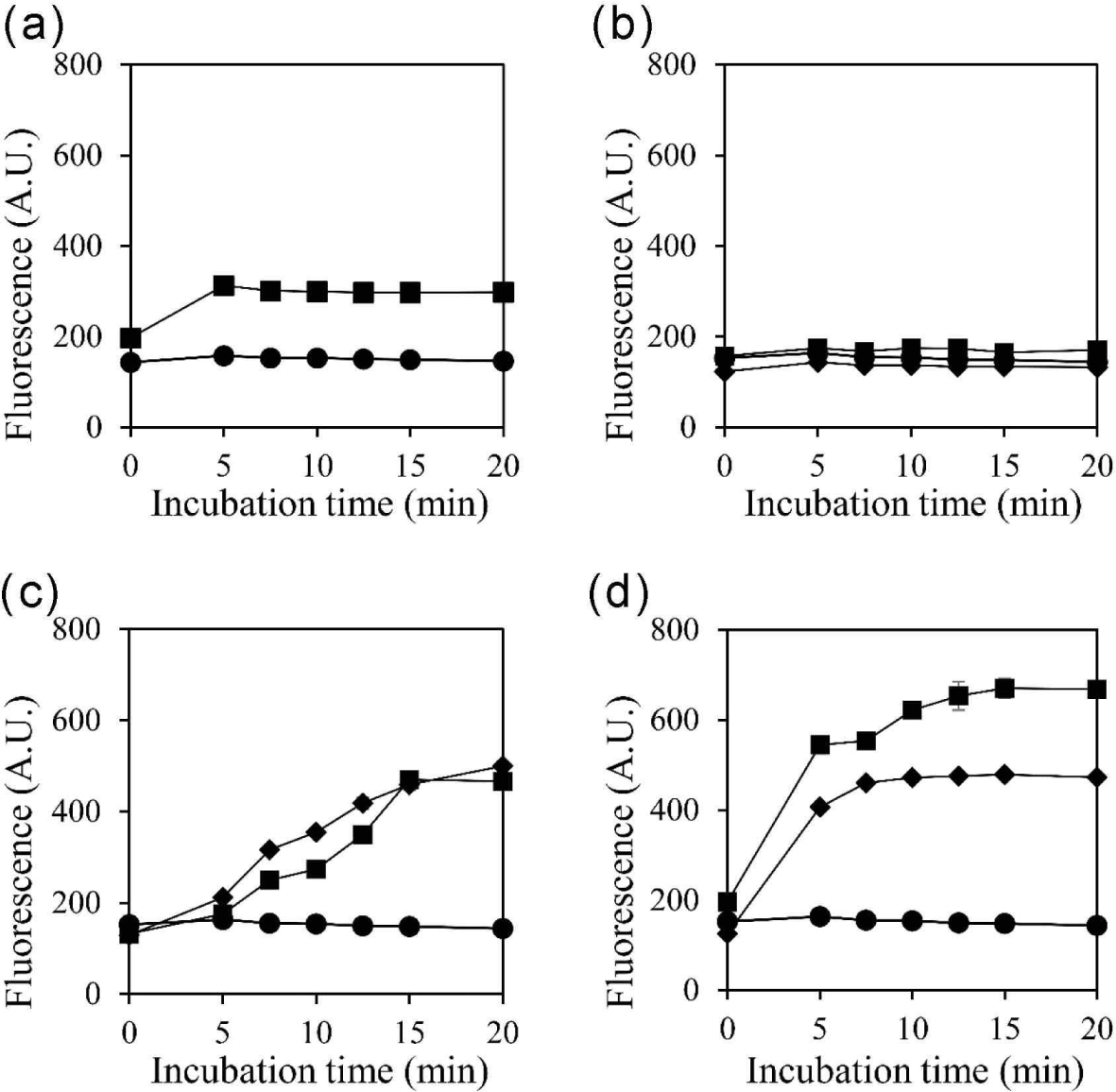
Previous studies have demonstrated the effectiveness of membrane-damaging agents in reducing persister cells (Dombach et al., 2021; Grassi et al., 2017), and recent studies have documented the ability of fatty acids to penetrate the cell membrane (DeMars et al., 2020). Here, we measured the effect of selected substances on cell membrane permeability (Fig. 3). Exogenous, heterogeneous fatty acids may alter the membrane properties (DeMars et al., 2020). In turn, an increase in cell membrane permeability may offer a means to eliminate persister cells (Defraine et al., 2018). In line with the results in Fig. 1, Coicis Semen extract, oleic acid, and linoleic acid increased cell membrane permeability, whereas palmitic acid did not (Fig. 3). An increase in cell membrane permeability could facilitate the entry of antimicrobial substances into the cell (Brun et al., 2018), leading to the disruption of cell integrity. Additionally, given the nature of persister cells where metabolism is halted, repairing the disrupted cell membrane is presumed to be challenging. Therefore, we propose that the inhibitory effect of Coicis Semen extract on persister cells is associated with changes in membrane permeability.
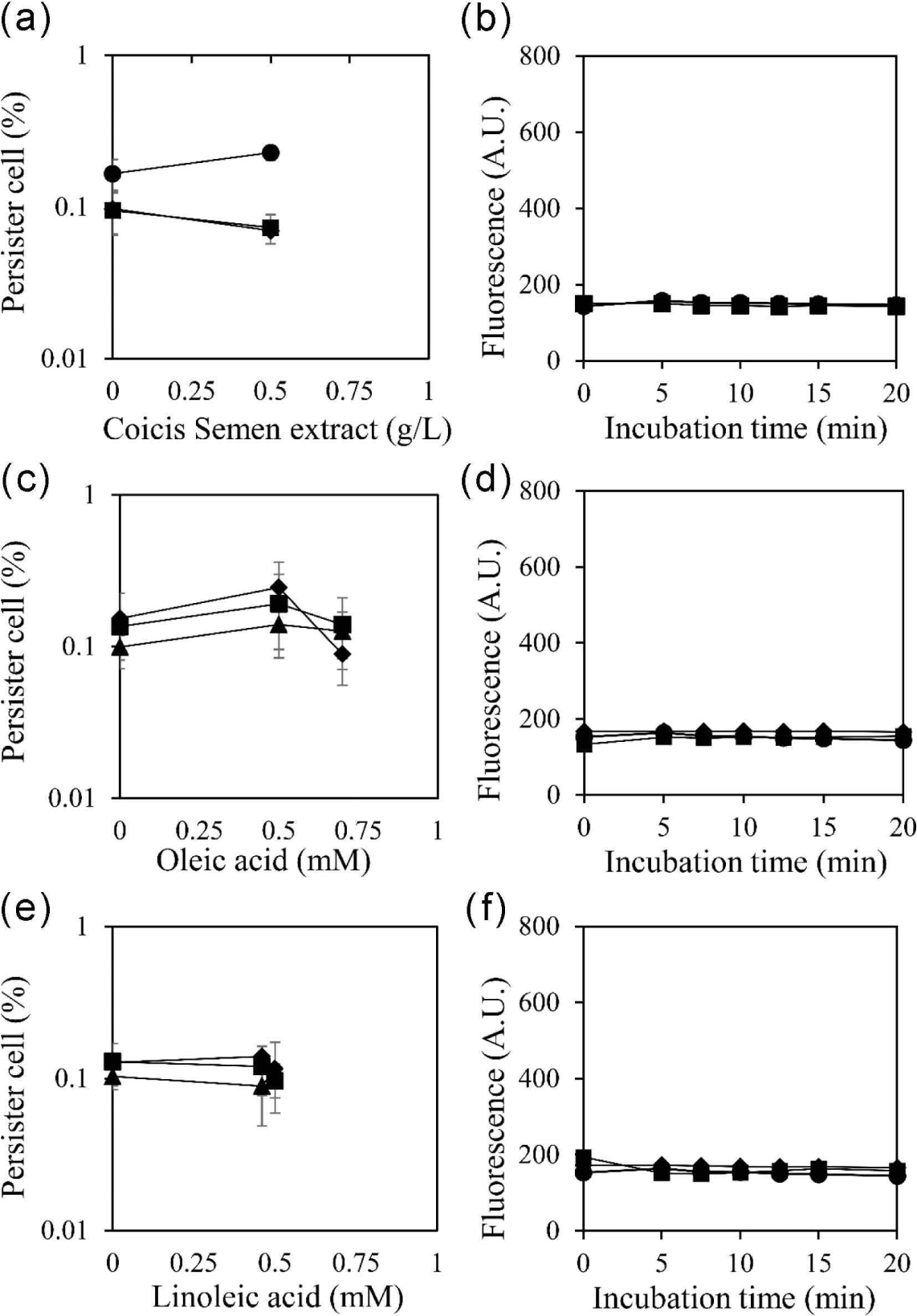
The hydrophobic nature of fatty acids is suggested to be a crucial factor in enhancing the permeability of cell membranes. To validate this hypothesis, the reduction of persister cells and the increase in cell membrane permeability were evaluated with a surfactant, 1% Tween 80 (Fig. 4). The results demonstrated that the increased permeability of the cell membrane was abolished by 1% Tween 80. This outcome suggests that the hydrophobic properties of oleic acid and linoleic acid are essential for entering the cell membrane and increasing membrane permeability, thereby reducing the occurrence of persister cells. The simultaneous abolishment of both the reduction of persister cells and the increase in membrane permeability by Tween 80 suggests a close interrelation between them.
4. CONCLUSIONS
In this study, we explored the impact of Coicis Semen, a medicinal herb in the Asian region, on persister cell reducing activity in S. aureus. Coicis Semen demonstrated the ability to decrease the number of persister cells by disrupting the bacterial membrane. These findings highlight the potential of Coicis Semen as an adjunct in the antimicrobial treatment of S. aureus infections, offering a means to reduce persister cells and significantly curbing recurrence and the emergence of resistant strains. The discovery of novel and effective strategies to diminish persister cells is crucial for the treatment of bacterial infections, and the utilization of natural products such as Coicis Semen may enhance the efficacy of antibiotics.
