1. INTRODUCTION
The back-to-nature style of using natural instead of synthetic dyes has made great contributions to sustainability and consumer habits. The food industry prefers natural over synthetic dyes. However, the textile industry does not follow this behavior due to the price aspect. Even restrictions cannot hinder the application of synthetic dyes in the textile industry (Kiumarsi et al., 2017; Parvinzadeh Gashti et al., 2014). Therefore, scientists and researchers should consider competitively priced natural dyes to maintain ecofriendliness and sustainability.
Processing waste into products decreases the prices because raw materials are inexpensive. Additionally, this approach improves the circular economy and sustainability of products. Although tannins are compounds commonly used as adhesive materials, their use as natural dyes is often ignored (Hendrik et al., 2019; Trisatya et al., 2023); they are sustainable and ecofriendly alternatives to synthetic dyes and provide the opportunity to blend the beauty of natural colors with improved environmental values across various industries.
Another advantage of natural dyes developed from waste or by-products is their abundant resources. Natural dyes can be derived from different plant, animal, and fungal parts and minerals (Haji et al., 2016; Ju and Roh, 2020; Rahayuningsih et al., 2019; Roh and Jo, 2022). Indonesia has great potential natural resources for generating natural dyes. Merbau (Intsia bijuga), an unexplored resource, can be utilized as a natural-dye source, with a brown color, produced by tannins (Malik et al., 2016). Further, tannins are not limited to natural dyes but have broad applications as coagulants, superplasticizers, floating agents, and adhesives (Fraga-Corral et al., 2020). The structures of several forms of tannins are shown in Fig. 1.
Merbau is primarily used as hardwood for furniture, musical instruments, and construction. The wood processing industry emits 60% of underdeveloped or underutilized wastes or residues (Prasetya et al., 2021). However, the extraction of tannins from merbau has obstacles, such as low resource access and research interest, that impede development. Wood waste or residue is primarily processed into firewood without any other processing steps. Hence, its value does not increase. Rahayuningsih et al. (2011) compared mahogany (Swietenia mahagoni) and merbau sawdusts as prospective raw materials for natural dyes and found that the durability, quality, and extraction of merbau were superior to mahogany (Rahayuningsih et al., 2011). Malik et al. (2016) successfully extracted tannins from merbau wood using water, ethanol, and ethyl acetate with yields of 1.34%, 12.45%, and 12.56%, respectively (Malik et al., 2016). Polar solvents provide high yields in tannin extraction. However, their use increases the carbon footprint of the natural-dye product. Ease and safety issues are other reasons for using water as an extraction solvent. Mun et al. (2020, 2021) investigated the extraction of Pinus radiata bark with 0.8% NaHCO3 aqueous solution and prepared a neutral extract that was used as a natural dyestuff for cotton and silk fabrics (Mun et al., 2020, 2021). Jung et al. (2017) extracted phenolic compounds from oak wood (Quercus mongolica) through steam explosion. They identified the optimal extraction conditions using response surface methodology (RSM; Jung et al., 2017). Jung and Yang (2018) employed RSM to optimize a two-stage extraction process that consisted of steam explosion and water extraction (Jung and Yang, 2018). The study conducted by Um et al. (2020) aimed to investigate the optimization of ascorbic acid extraction from Rugosa rose (Rosa rugosa Thunb.) fruit by the utilization of RSM (Um et al., 2020). RSM was used to optimize mycelial growth and cordycepin content in the submerged culture of Paecilomyces japonica (Ha et al., 2020).
Solid–solvent extraction depends on solvent type, particle size, solid–solvent ratio, and temperature. This study aims to identify the optimal extraction condition that provides the highest tannin concentration in extracts. In this study, water was chosen as the solvent. Meanwhile, particle size was defined in accordance with the available sawdust, and solid–solvent ratio (merbau sawdust–water) and temperature were varied to identify the optimal extraction condition. The optimal condition and design parameters for upscaling extraction were used as response parameters. This study also performed a simple examination of the extraction mechanism.
2. MATERIALS and METHODS
Merbau sawdust was obtained from the Indonesia Natural Dye Institute Research Center, Universitas Gadjah Mada. Distilled water was used as a solvent for extraction process, and ethanol was utilized as a solvent for total tannin content analysis (Soxhlet method). The reagents used for tannin analysis were sulfuric acid, potassium permanganate (KMnO4), and indigo carmine (C16H8N2Na2O8S2), procured from Sigma-Aldrich (St. Louis, MO, USA).
Merbau sawdust was preliminarily dried in an oven at 110°C to a constant weight at which the sample had the minimum water content necessary to generate the optimal conditions for extraction. Merbau sawdust was sieved with a 45/60 mesh to obtain homogenous particle sizes.
Merbau sawdust was extracted through Soxhlet and shaking water bath methods. The Soxhlet method was used to obtain the total tannin content of the sawdust. A total of 3 g of sawdust was covered with filter paper and soaked in 500 cm3 of ethanol (96 wt%) until a clear solvent was obtained. Meanwhile, the shaking water bath method was implemented for 3 h using water and various independent variables (Table 1). Both solutions were analyzed through the volumetric method to obtain tannin concentrations.
| Independent variable | Range of level | ||||
|---|---|---|---|---|---|
| –1 | –δ | 0 | 1 | 2 | |
| Temperature, °C (X1) | 30 | - | 45 | 60 | - |
| Solid: solvent ratio, (X2) | 0.083 | 0.130 | 0.167 | 0.250 | 0.333 |
The tannin extract solution (50 cm3) was added to 50 cm3 distilled water and boiled until its volume decreased to 50 cm3. A total of 5 mL solution was mixed with 20 cm3 indigo carmine solution in 200 cm3 volumetric flask, with distilled water and titrated with 0.1 N KMnO4 standard solution. Volumetric analysis was conducted twice on every sample. Meanwhile, a blank solution, without any sample, was prepared and titrated correspondingly from the indigo carmine solution. Tannin yield was calculated using Equation (1).
Where C is the tannin content (g/g sawdust); Vs is the titrated volume of the sample; Vb is the titrated volume of the blank; DF is the Dilution factor; ms is the weight of merbau sawdust.
Indigo carmine solution was prepared by dissolving 3 g of indigo carmine (analytical grade, Sigma-Aldrich) in 100 mL of distilled water with continuous heating. The boiled solution was cooled and mixed with water and 25 cm3 sulfuric acid in a 500 cm3 volumetric flask.
The independent variables used to identify the optimal extraction conditions are shown in Table 1. The response variables (dependent variables) in volumetric analysis were analyzed using RSM with Minitab® 19.
Tannin extraction is microscopically regarded as the mass transfer of tannins from inside sawdust solids into solvents. The mechanism of mass transfer is theoretically described as the diffusion of tannin molecules in sawdust solids to the interface of solid particles, transfer of tannin molecules from the particle surface to the liquid film at the particle surface, and convective mass transfer of tannins from the liquid film into the bulk liquid solvent. This study proposed two mass transfer models.
This model proposes that the diffusion rate in solid particles and convective mass transfer at the particle surface control extraction. Mass transfer is based on Fick’s law of diffusion and convection:
This model is based on several assumptions: sawdust particles are spherical solids; the process is isothermal; volume is fixed throughout the experiment; a concentration gradient does not exist in the bulk solvent. Equation (4) was obtained on the basis of the mass balance analysis of the solid volume element:
This equation can be solved numerically by using the initial and boundary conditions specified below:
The value of CAf was generated by compiling the mass balance of solutes in the solid and liquid phases:
In this case, De is the diffusivity coefficient of the solid (cm2/min), CA is the tannin concentration (g/cm3) in sawdust at time t (min), CAo is txhe tannin concentration in sawdust at t = 0, (g/cm3), CAf is the tannin concentration (g/cm3) in the solvent at time t, C*Af is the tannin concentration (g/cm3) in the solvent phase that is in equilibrium with the tannin concentration at the solid particle surface, CAfo is the tannin concentration (g/cm3) in the solvent phase at t = 0, kc is the mass transfer coefficient (cm/min), kcα is the volumetric mass transfer coefficient, 1/min, α is the mass transfer specific surface (area/volume) of sawdust (cm2/cm3), VS is the solid volume (cm3), V is the solvent volume (cm3), r is the radial position in the sawdust particle from the center (cm) and R is the sawdust radius (0.009 cm).
The equilibrium between solid and liquid concentrations at the solid–liquid interface is expressed as
where kH is the equilibrium constant. In this case, kH is considered constant across various tannin concentrations.
The second model assumes that the diffusion rate inside sawdust particles is faster than that of particle surfaces. This assumption is based on the fact that sawdust particles are tiny, with a radius of only 0.009 cm, the extraction time to reach equilibrium is < 3 h, and sawdust wood is microscopically porous with 0.833 g/cm3 density. This assumption accounts for the rapid diffusion rate in the solid phase. Therefore, the second model assumes that the tannin concentration inside sawdust particles is always homogeneous regardless of position and that mass transfer at particle surfaces controls extraction.
The equilibrium equation is then estimated with
where C*Af is the tannin concentration in the solution (in equilibrium with tannins in the solid, g/mL), XA is the tannin concentration in sawdust solid particles (g tannin/g solid), and KHs is the equilibrium constant. The correlation of KHs in Equation (10) with the equilibrium kH of Model 1 in Equation (9) is given by with ρs = wood density (0.83 g/cm3).
The mass transfer of tannins in the solution is formulated as
where As is the total surface area of sawdust particles in the solution. During extraction time t = t0, the concentration of tannins in the solid and solution are XA0 and CAf0. The overall mass balance of tannins in the extraction mixture yields the following equation:
where Ms is the total mass of sawdust solids. The solution and integration of Equations (12) and (13) result in
where and . In this case, XAin is the tannin concentration in sawdust at the beginning of extraction (t = 0). Equation (14) is written in another form for evaluation:
KHs and kc are evaluated through the minimization of the sum of squares of errors (SSEs).
The parameters of mass transfer in both models are evaluated through the minimization of SSE, in which error is defined as the difference between the experimental data and model prediction of CAf:
The Van’t Hoff equation describes the correlation of the solid–liquid equilibrium constant kH described in Equation (17) with the extraction temperature in isothermal processes.
where H is the enthalpy (kJ), S is the entropy (kJ/K), R is the gas constant (J/mol·K), and T is the temperature (K).
3. RESULTS and DISCUSSION
The total tannin content of merbau sawdust was determined through the Soxhlet method, and a tannin content of 0.3472 g tannins/g merbau was obtained. Volumetric analysis was performed to identify the value of equivalent tannins and all tannin components, such as (–)-robidanol, robinetin, catechin, naringenin, dihydromyricetin, piceatannol, quercetin, and fustin (Sari et al., 2021).
In Fig. 2, tannin concentration and yield are presented as a function of solid–solvent ratio. Solid–solvent ratio had a considerable effect on tannin concentration but not on yield. This result was reasonable because tannin concentration, which increased with variables, was calculated as the amount of extracted tannins divided by the total amount of solvent, which was a fixed value. Meanwhile, yield was divided by the solid amount, which increased with variables.
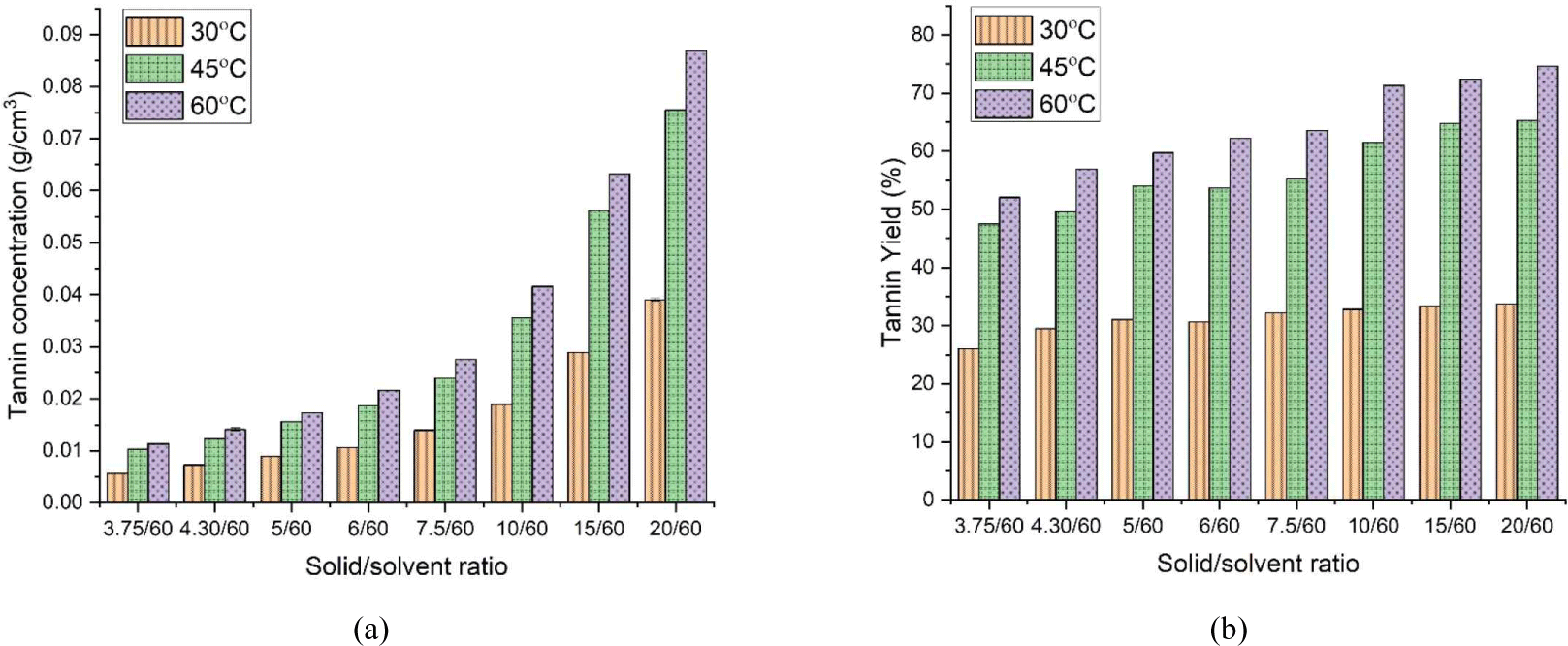
Temperature significantly affected both parameters. The increase in extraction temperature accelerated tannin extraction from merbau sawdust. It was primarily affected by the mass transfer coefficient, which was a function of temperature.
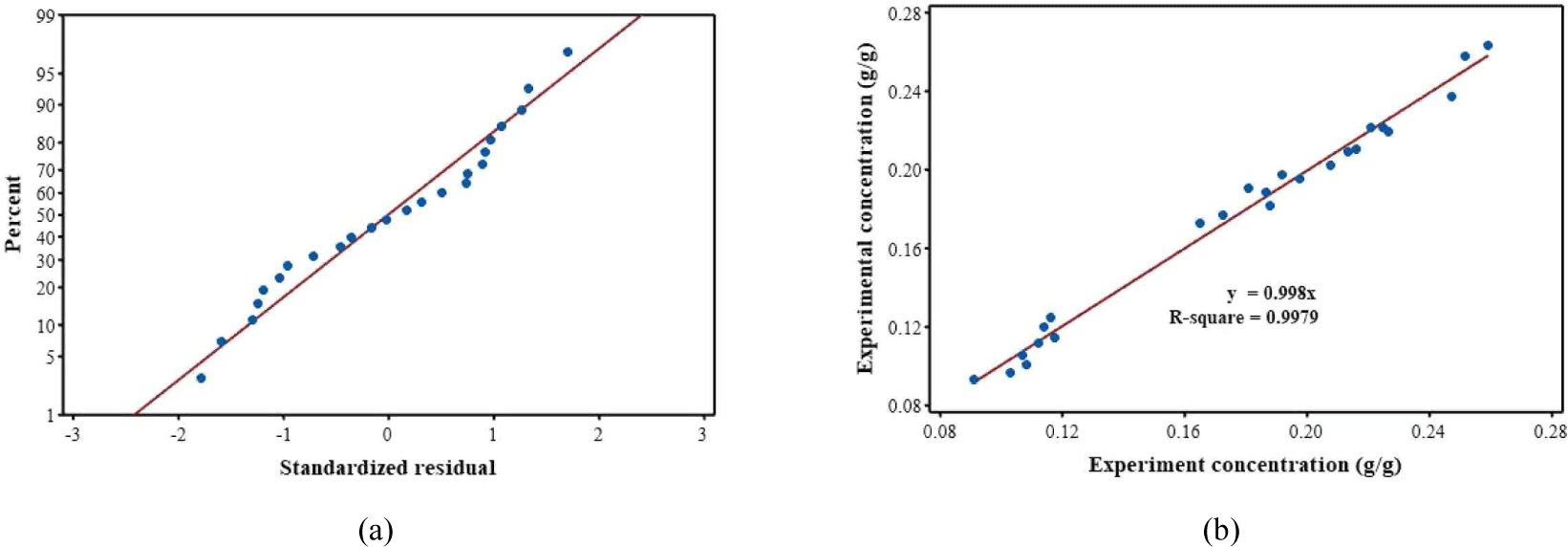
Experimental results were analyzed using Minitab© 19 to investigate the effects of temperature and solid–solvent ratio on extracted tannin concentration. Table 2 shows constants and F- and p-values. The constants describe the magnitude of values in the nonlinear polynomial equation of RSM results (Bezerra et al., 2008; Mansour et al., 2017; Montgomery, 2017). F- and p-values can describe model fitness (Rahayuningsih et al., 2021). A higher F-value than F-statistic indicates an acceptable model. Meanwhile, p-value of the model (0.000) should be < 0.05 to be statistically significant. R-square of the model was 0.9884.
Further analysis showed that residual values increased with increasing model accuracy [Fig. 3(a); Agarry and Ogunleye, 2012; Mohajeri et al., 2010]. Data were normally distributed. The plot of the experimental and modeled concentrations are shown in Fig. 3(b) with gradient and R-square values ~1.00.
The effect of independent variables is illustrated in Fig. 4. Temperature and solid–solvent ratio positively affected tannin concentration [Fig. 4(a)]. Increasing temperature led to the softening and expansion of solid substances along with an increase in diffusivity and solubility of tannins (Sun et al., 2011). However, both parameters stabilized at some point with the further increase in tannin concentration. Meanwhile, the combination of temperature and solid–solvent ratio resulted in a high positive value of tannin concentration [Fig. 4(b)].
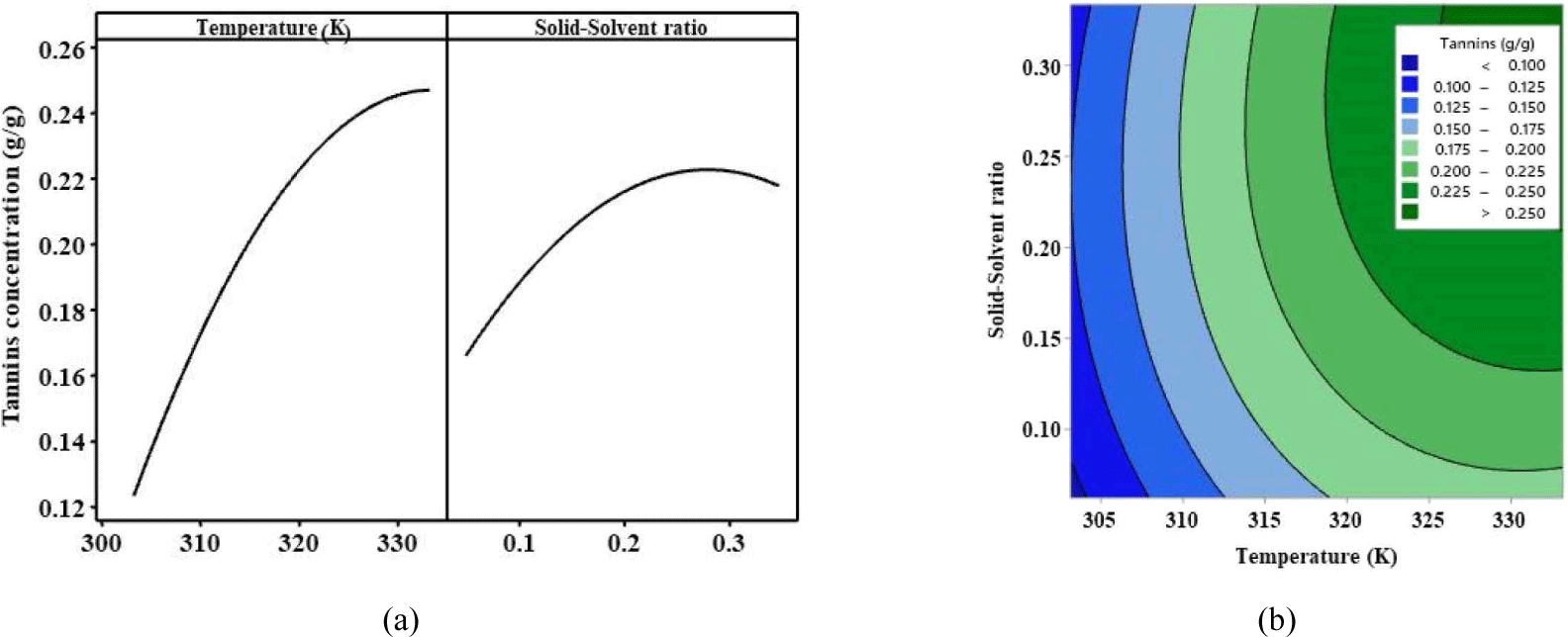
A final analysis was conducted using RSM to identify the optimal extraction condition. The optimal condition was identified as a temperature of 333.15 K and solid–solvent ratio of 0.3224 and yielded 0.2639 g tannins/g merbau. However, the experiment showed that yield decreased when the solid–solvent ratio further increased because the solution was absorbed and trapped in solid pores. This phenomenon affected the optimal extraction condition. The optimal solid–solvent ratio of 0.125 resulted in a yield of 0.2217 g tannins/g merbau. Nevertheless, the optimal modeling condition can still be implemented in industrial processes because the solid (merbau sawdust) and solution can be separated using a filter press machine.
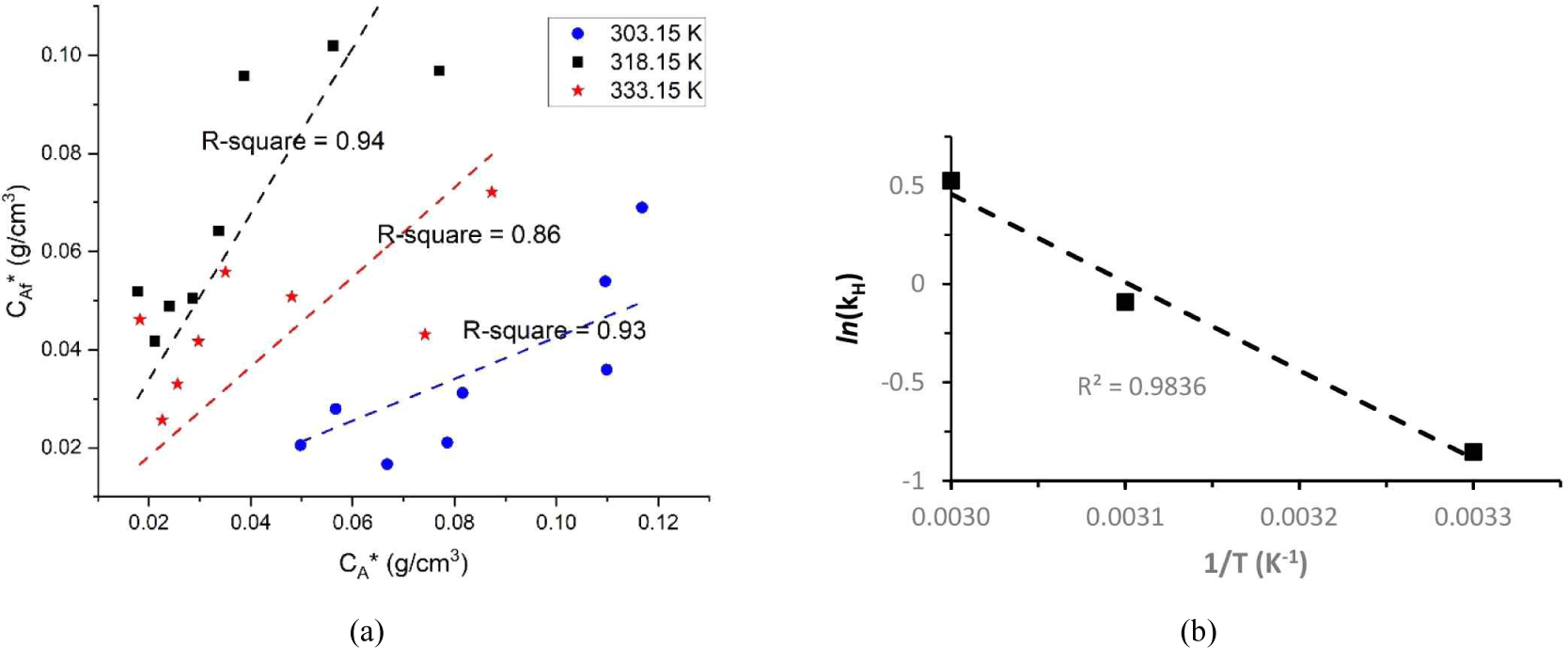
The equilibrium constant at various temperatures was calculated using Equations (9) and (17). Fig. 5(a) and (b) shows that kH values at 303.15, 318.15, and 333.15 K were 0.4254, 0.9129, and 1.6908 (cm3/cm3), respectively. The equilibrium constant was obtained based on the slope under each condition. Consistent with the findings of previous research, the results of this work showed that high temperatures were associated with high equilibrium constants. Next, the obtained equilibrium constant was utilized in Equation (9) to generate a temperature function. The values of △H and △S were generated in accordance with Equation (17) by recalculating the linear regression and were 38.7 kJ and 120.53 kJ/K, respectively. Therefore, spontaneous and endothermic processes occurred during the solid–liquid extraction of merbau sawdust.
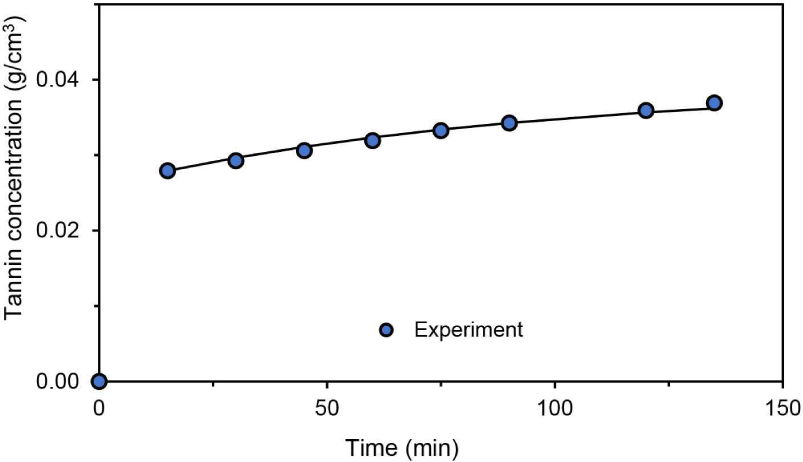
Models 1 and 2 were evaluated based on the tannin concentration obtained at 60? as a function of time. The mass transfer coefficient (kca) and diffusivity (Dein Model 1 were evaluated using Equations (4), (5), (6), (7), and (8) by applying the finite difference approximation method.
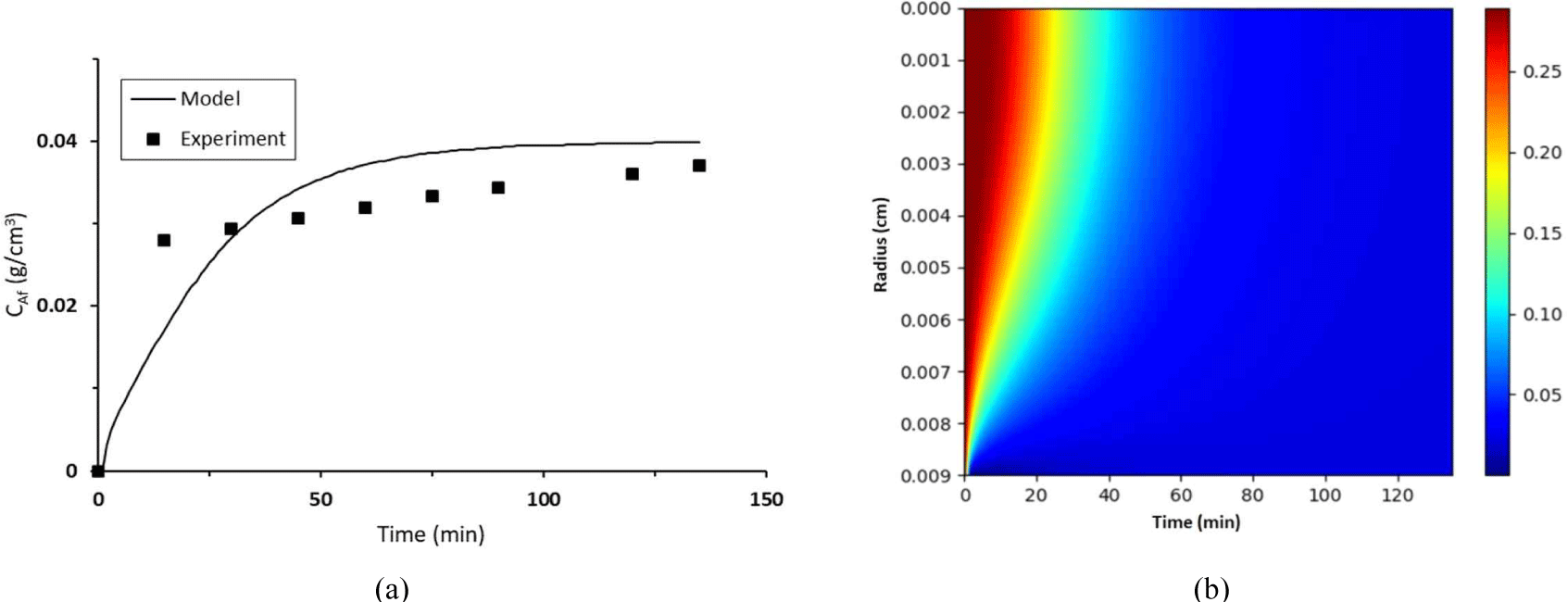
The concentration profile is presented in Fig. 6. The model and experimental CAf profiles are compared in Fig. 6(a), which confirms that the presence of strong driving forces at the early stage of extraction resulted in rapid diffusion. Meanwhile, Fig. 6(b) presents the tannin concentration around merbau sawdust particles. These findings will help scholars develop a green tannin extraction process. However, stabilizing tannin content, structure, and activity remains a challenge in tannin extraction (Cuong et al., 2020).
Moreover, tannin amount decreased with prolongation of the handling time of raw materials (Cuong et al., 2020). Thus, the local raw material plant is considered the best option for tannin production. Meanwhile, process ease and solvent availability are other factors to be examined.
The initial values of mass transfer coefficient (kc) and diffusivity (De) were estimated and subsequently computed by using the Hooke–Jeeves technique until their minimum SSEs were obtained. The results are presented in Fig. 6. The value of De is 3.71 × 10–7 cm2/min and kc is 0.681 cm/min respectively, with SSE of 2.317 × 10–4. The diffusivity coefficient obtained in this work was lower than previously reported values of 1.69 × 10–6–1.86 × 10–6 (Tasheva et al., 2019) and 1.23 × 10–5 cm2/ min (Stefanova et al., 2017) because this experiment used water as the solvent in contrast to other studies that used ethanol. However, the effective diffusion coefficient was greater than 6.24 × 10–8–2.22 × 10–7 cm2/min (Simeonov and Koleva, 2012). Raw material properties (tannin content, structure, and activity; particle size; porosity; water content) and extraction process parameters (method, temperature, and time) accounted for differences in the effective diffusion and mass transfer coefficients.
For Model 2, calculation with Equation (15) and SSE minimization resulted in KHs = 1.16 (equivalent to kH = 0.718), kc = 2.15 × 10–5 cm/min, and SSE = 1.2 × 10–6. The mass transfer coefficient of Model 2 was very small. Fig. 7 shows CAf as a function of time. In this figure, the calculated CAf was in line with experimental CAf. It is clear that the calculated values of CAf were close to experimental values. Furthermore, SSE of Model 2 was smaller than that of Model 1.
Therefore, the mass transfer rate was controlled by the transfer of tannins at the interface of sawdust particles, and diffusion in solid particles was rapid due to the porosity of solid sawdust.
4. CONCLUSIONS
Merbau sawdust can be processed and utilized as a natural brown-colored dye. The ecofriendly extraction of natural dye from merbau sawdust was successfully conducted with water as the solvent instead of an organic solvent. The yield of 0.2217 g tannins/g merbau was obtained under the optimal conditions of 333.15 K and solid–solvent ratio (merbau sawdust–water ratio) of 0.125. Temperature and solid–solvent ratio had a positive effect on tannin yield. Extraction was controlled by convective mass transfer at the interface of solid particles. The obtained parameters can be implemented to design the industrial extraction of natural dyes with water as an ecofriendly solvent.