1. INTRODUCTION
Yersinia enterocolitica is the causative agent of bacterial gastroenteritis (Riahi et al., 2021), specifically yersiniosis (Carniel and Mollaret, 1990), which produces symptoms of fever and abdominal pain. However, it can also cause serious infectious diseases, such as myopericarditis (Nehoff et al., 2022), endocarditis (Ioannou et al., 2021), and autoimmune thyroid disorders (Zangiabadian et al., 2021). In general, yersiniosis heals spontaneously, however, in children, it may produce more serious symptoms (CDC, 2016a) such as diarrhea, which is often bloody, although it can be cured by antibiotics (CDC, 2016b). Y. enterocolitica can grow at low refrigerator temperatures (Palonen et al., 2010), and thus it is an enteric infectious disease that has spread because of the use of refrigerators. The Centers for Disease Control and Prevention of the United States estimates that each year in the United States alone 117,000 people become infected, 640 hospitalized, and 35 die from this disease (CDC, 2016c).
It is important to treat the disease after symptoms appear, but prevention is even more important. Prevention is more important in the management of yersiniosis because an effective treatment is possible when the diagnosis is clearly indicative of yersiniosis. Hygienic methods that are useful for controlling general bacterial infections may be applied for preventing yersiniosis as well. Additionally, contamination by Y. enterocolitica can be controlled by inhibiting the formation of biofilms. Y. enterocolitica is a bacterium that uses flagella to form biofilms (Kim et al., 2008). The presence of biofilms not only makes it difficult to physically remove the bacterial cells, but also increases their resistance to ethanol (Park et al., 2015), the most commonly used sterilizing agent. Inhibiting the formation of biofilms therefore provides an effective sanitary method to control bacterial contamination.
In this study, we identified natural products that can inhibit the formation of biofilms that are produced by Y. enterocolitica. Plant natural products, including forest products, contain compounds with various biological functions (Adfa et al., 2020; Ahn et al., 2021; Arsyad et al., 2020; Ham and Kim, 2019; Huh et al., 2022; Lee et al., 2021; Na and Kim, 2022; Nkogo et al., 2022; Oramahi et al., 2022; Yang et al., 2022; Yoon and Kim, 2021). The library of extracts used in this study is a collection of extracts of plants that include forest products that are used as foods or herbal medicines, and since they are natural materials, they are considered to be safe. If an edible plant extract is used as an inhibitor of the formation of biofilms produced by Y. enterocolitica, it can be used in foods that are expected to be contaminated with Y. enterocolitica within a concentration range that does not significantly change the flavor or preference of the food, and it can be applied as a coating agent for packaging materials, or as a disinfectant on the food surface.
2. MATERIALS and METHODS
The methanol extract library that was used in this study was prepared in a previous study (Ham and Kim, 2018). The distilled water, 50% ethanol, and 95% ethanol extracts of selected edible plants were prepared as previously described (Ham and Kim, 2018).
Powdered extracts were dissolved in the extract solvent at a concentration of 50 g/L and the undissolved particles were removed by centrifugation at 12,000×g for 20 min and then filtered using a syringe filter (catalog number: FJ25ASCCA002DL01, GVS Korea, Namyangju, Korea). The extract library was stored at –80°C.
The following media were prepared as previously described (Kim et al., 2008). LB agar plates were prepared with 25 g/L LB broth (REF: 244620, Becton Dickinson Korea, Seoul, Korea) and 15 g/L agar. TYE broth containing 10 g/L tryptone (REF: 211705, Becton Dickinson Korea) and 5 g/L yeast extract (REF: 212750, Becton Dickinson Korea), and TYE plates were prepared using TYE broth and 15 g/L agar. M63 glucose medium (M63GM, 1 L) contained 200 mL of ×5 M63 medium (15 g/L KH2PO4, 35 g/L K2HPO4, 10 g/L (NH4)2SO4, 2.5 mg/L FeSO4), 10 mL of 20% glucose, and 1 mL of 20% MgSO4 in distilled water.
Y. enterocolitica (KCCM 41657) cultures were obtained from the Korean Culture Center of Microorganisms (Seoul, Korea) and were stored at –80°C. The cells were streaked on LB plates and grown at 26°C for 2 days to obtain a single cell colony. The single cell colony that was obtained was streaked on fresh TYE plates and grown at 26°C for 2 days to obtain a second-round single cell colony. The newly obtained single cell colony was inoculated into 5 mL of TYE broth then incubated overnight at 26°C to obtain a pre-culture. Pre-cultures were inoculated into each well of a 96-well polyvinyl chloride (PVC) plate at an Abs600 of 0.05 in 100 μL of M63GM. The cells were incubated for 24 h at 26°C. Cell growth was measured by Abs595 before quantification of biofilm formation using a microplate reader (Opsys MR™ microplate reader, Dynex, Incheon, Korea).
The biofilm that was produced was evaluated quantitatively using crystal violet as previously described (Kim et al., 2008). Cell growth was evaluated by measuring Abs595 of the culture before applying crystal violet. Planktonic cells were rinsed with water, and 100 μL of 1% crystal violet was added to each well and incubated at room temperature for 15 min. The wells were then rinsed three times with water, and 100 μL of 95% ethanol was added to each well. After allowing the crystal violet to dissolve in the biofilm for 15 min, the absorbance was measured at 595 nm using an Opsys MR™ microplate reader. Inhibition of biofilm formation was calculated by the following Equation.
Abs595, CV-S is the amount of biofilm produced by the extract and Abs595, CV-0 is the amount of biofilm produced without extract, 0 g/L of extract. The inhibited biofilm formation was expressed as a positive value and the enhanced biofilm formation was expressed as a negative value. All biofilm amounts were expressed as Abs595 after crystal violet staining. The values of mean and SD were calculated using six results.
3. RESULTS and DISCUSSION
The inhibitory activities of the various extracts from the 140 methanol extract library described previously (Ham and Kim, 2018) on the formation of Y. enterocolitica biofilms were evaluated. The extracts were cultured for 24 hours at a concentration of 0.5 g/L and 12 extracts that inhibited biofilm formation by more than 70% were selected (Fig. 1). The selected plants were Agastachis Herba, Agrimoniae Herba, Diospyros kaki leaves, Elsholtziae Herba, Ginkgonis Semen, Lycopi Herba, Melonis Pedicellus, Menthae Herba, Mori Radicis Cortex, Polygoni Multiflori Radix, Prunellae Spica, and Schizonepetae Spica. At a concentration of 0.12 g/L, all extracts exhibited similar results compared to the maximum inhibitory effect that was obtained at 0.5 g/L. The extracts of Prunellae Spica and Schizonepetae Spica showed strong inhibitory activity.
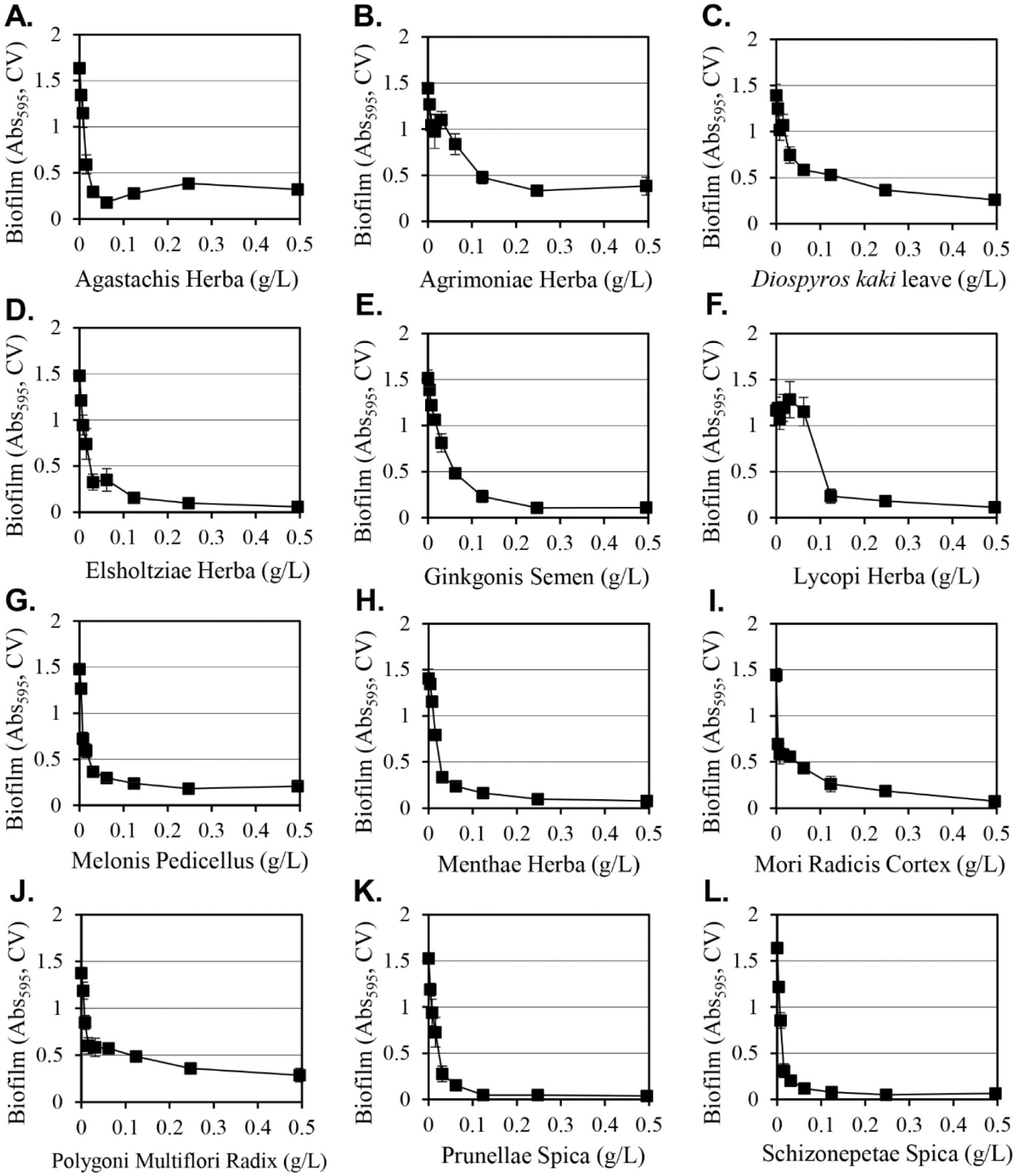
The inhibition of cell growth was evaluated in cultures that were under the same conditions for biofilm formation (Table 1). The 12 extracts that were selected all inhibited growth, although there was a difference in the degree of inhibition. At 0.5 g/L, the Menthae Herba extract produced the lowest level of inhibition (14%), while the Melonis Pedicellus extract produced the greatest amount of inhibition (71%). The inhibition of cell growth can reduce the amount of biofilm. When comparing the inhibition of biofilm formation with the inhibition of cell growth, the Melonis Pedicellus extract at 0.5 g/L reduced biofilm formation by 86% and cell growth by 71%, which suggests that a significant portion of the inhibition of biofilm formation is due to the inhibition of cell growth. However, in the case of the Menthae Herba extract, biofilm formation was decreased by 94% and cell growth was decreased by 14%, suggesting that the inhibition of biofilm formation is not only due to simple cell growth inhibition, but also a biological mechanism that selectively inhibits only biofilm formation by Menthae Herba extracts. The growth-inhibiting effect provides a more effective method of cell removal because the inhibition of biofilm formation is directed at the removal of Y. enterocolitica to promote good hygiene.
Methanol as a solvent is effective at extracting a wide range of hydrophilic and hydrophobic substances from plants, but it cannot be used to manufacture edible products due to safety concerns. Since Y. enterocolitica causes gastroenteritis by the ingestion of contaminated food, water and ethanol extracts of 12 selected plants that can be applied as food were prepared and their inhibitory activities on the formation of biofilms were evaluated (Table 2). Among the extracts prepared using water, 50% ethanol, or 95% ethanol, the 50% ethanol extract consistently exhibited strong inhibitory activity against the formation of biofilms. When 0.5 g/L of 50% ethanol extracts were used, Lycopi Herba, Melonis Pedicellus, and Agastachis Herba extracts produced 15%, 27%, and 37% inhibition of biofilm formation, respectively, and their strong biofilm formation inhibitory activity of methanol extracts was eliminated. Diospyros kaki leaves, Elsholtziae Herba, Mori Radicis Cortex, Prunellae Spica, Agrimoniae Herba, Menthae Herba, Schizonepetae Spica, and Ginkgonis Semen extracts demonstrated reduced inhibitory activity when considering the biofilm formation inhibition change according to the dilution of the extract (Fig. 2). The 50% ethanol Polygoni Multiflori Radix extract was the only extract that produced a stronger inhibitory effect on biofilm formation than methanol extracts. The Polygoni Multiflori Radix 50% ethanol extract inhibited biofilm formation by 99.7% at 0.5 g/L, and it produced an inhibitory effect of 85% at 0.13 g/L.
InB: Inhibition degree of biofilm formation (%) calculated according to the formula in ‘Materials and Method’ section.
InC: Inhibition degree of cell growth (%) = (Abs595,0 – Abs595,S) / Abs595,0 × 100. The calculation was similar with inhibition of biofilm formation except for switching Abs595, CV-0 to Abs595,0 and Abs595, CV-S to Abs595,S. Abs595,S was the cell growth by the extract and Abs595,0 is the cell growth without extract, 0 g/L of extract.
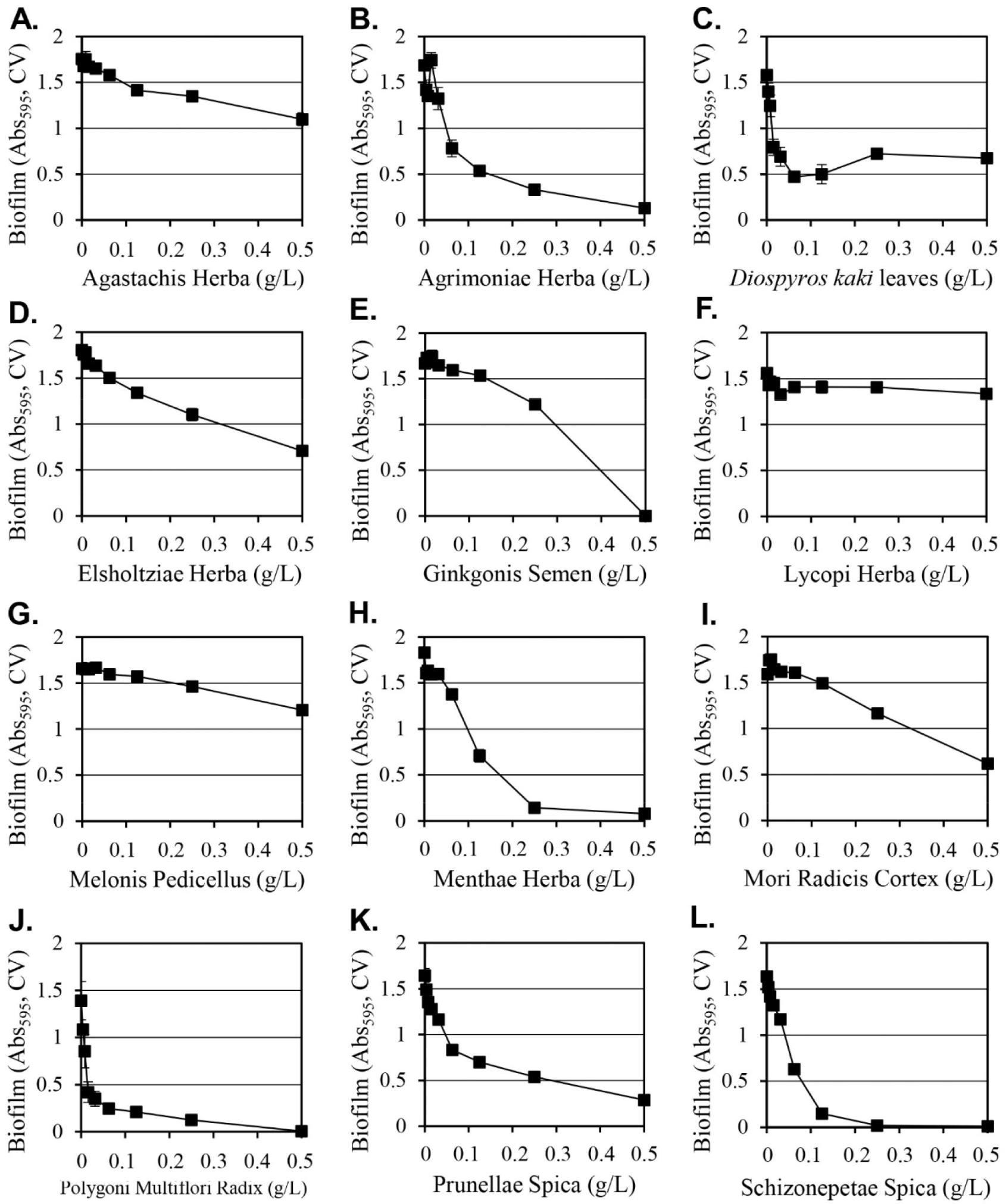
The 50% ethanol extract of Polygoni Multiflori Radix inhibited cell growth by 73% at 0.5 g/L of, thus a significant portion of the inhibitory activity on biofilm formation was attributed to the inhibition of cell growth. Agrimoniae Herba and Diospyros kaki leaf extracts exhibited cell growth inhibitory activities of 71% and 66%, respectively. Ginkgonis Semen, Lycopi Herba, and Mori Radicis Cortex extracts produced growth inhibition of 30%, 29%, and 25%, respectively, and the remaining six extracts (Menthae Herba, Agastachis Herba, Melonis Pedicellus, Schizonepetae Spica, Elsholtsiae Herba, and Prunellae Spica) produced similar or increased cell growth effects, although biofilm formation was inhibited. The 50% ethanol extract of Schizonepetae Spica inhibited biofilm formation by 99.4%, despite a 23% increment in cell growth, and 50% ethanol extracts of Menthae Herba inhibited biofilm formation by 96%, even though it increased cell growth by 30%. This suggests that these two extracts have a biological mechanism that specifically inhibits the formation of biofilms that is independent of cell growth.
Bacterial biofilm formation could be inhibited by interfering quorum sensing system, interfering the interaction between bacterial surface and biofilm-forming surface, interfering alarmone function, and interfering biofilm formation regulation mechanism (Srinivasan et al., 2021). The extracts presented in this study could inhibit the biofilm formation of Y. enterocolitica in a similar way.
In a previous study (Ham and Kim, 2018), it was shown that 9 methanol extracts among the 12 extracts presented in this study inhibited the biofilm formation of Streptococcus mutans, one of the causative bacteria of oral cavity. In this study, three extracts of plants, Ginkgonis Semen, Menthae Herba, and Prunellae Spica, were additionally presented for inhibition of biofilm formation in Y. enterocolitica. Hydro-distillated extract of Agastachis Herba showed a 0.021 g/L of minimum inhibitory concentration against Escherichia coli and inhibited its biofilm formation (Haiyan et al., 2016). The water extract of Agrimoniae Herba inhibited the biofilm formation of Pseudomonas aeruginosa by about 30% at the concentration range of 0.16–5 g/L, but did not inhibit the biofilm formation of Proteus mirabilis (Muruzović et al., 2016). In the case of Ginkgo biloba, Ginkgo Folium extract inhibited the biofilm formation of Salmonella spp. and Listeria spp. (Wu et al., 2016), but we cannot find any study on the inhibition activity of Ginkgonis Semen extract on the biofilm formation of bacteria. Previous studies showing directly inhibitory activity against bacterial biofilm formation by other extracts presented in this study were not found.
When comparing the biofilm formation inhibitory activity by the methanol extracts in Fig. 1 and that by the 50% ethanol extracts in Fig. 2, the five extracts of Agastachis Herba, Elsholtsiae Herba, Ginkgonis Semen, Lycopi Herba, and Melonis Pedicellus reduced the biofilm formation inhibitory activity significantly in 50% ethanol extracts. In previous studies, natural compounds, such as naphthoquinones (Di Marco et al., 2021), chlorogenic acids (Chen et al., 2022), and aporphinoid alkaloids (Di Marco et al., 2020), were known to inhibit the biofilm formation of Y. enterocolitica. The content of these compounds in the extract was changed by the solubility of these compounds in the extraction solvent, thereby changing the biofilm formation inhibitory activity of the extract.
In this study, substances that inhibit biofilm formation were identified from 140 edible plant extracts, which can be used to develop safe hygiene methods to prevent contamination of Y. enterocolitica.
4. CONCLUSIONS
In this study, extracts from 12 edible plants were demonstrated to inhibit the formation of biofilms by Y. enterocolitica, a food poisoning bacterium. The greatest inhibitory activity on biofilm formation was produced by 50% ethanol extracts of Polygoni Multiflori Radix. The 50% ethanol extracts of Schizonepetae Spica and Menthae Herba produced strong inhibition of biofilm formation in a growth-promoting environment, suggesting the existence of a biological mechanism that specifically inhibits the formation of biofilms. The use of extracts of edible plants as proposed in this study may lead to the development of a method for effectively preventing yersiniosis.
