1. INTRODUCTION
More than 30 species belonging to Ulmus genus grow in temperate regions of the Northern Hemisphere. Six species and two varieties of this genus are found in Korea: U. davidiana Planchon, U. davidiana Planchon var. japonica Nakai, U. laciniata Mayr, U. macrocarpa Hence, U. macrophylla Nakai, U. parvifolia Jacq.(U. coreana Nakai), U. pumila Linne (U. mandshurica Nakai) and Ulmus davidiana var. japonica for. Suberosa (Jung, 1974; Lee, 1996).
Flavan-3-ols are characteristic chemical components of Ulmus species (Son et al., 1989; Moon et al., 1995; Doskotch et al., 1973; Jung et al., 2007). Several interesting biological activities of flavan-3-ols including anti-inflammatory, anti-cancer, analgesic, antimicrobial, antineoplastic, anti-ulcer, antioxidant, antibacterial, anticancer and immunomodulatory properties have been reported previously (Hong et al., 1990; Park et al., 1996; Yang et al., 1996; Cho et al., 1996; Jun et al., 1998; Lee et al., 2000; Lee et al., 2001; Kim et al., 2002).
A flavan-3-ol compound, (+)-catechin 7-O-β.-D-xy-lopyranoside was isolated from the bark of U. Americana (Doskotch et al., 1973). In addition, (+)- catechin and (+)-catechin 5-O-β.-D-apiofuranoside from roots of U. davidiana were isolated (Son et al., 1989). Isoquercetin and rutin from leaves of U. parvifolia were isolated (Kim et al., 1992). Sterols, sterol glucoside and (+)-catechin 7-O-α-L-rhamnoside from barks of U. parvifolia were isolated (Moon et al., 1995). Sesquiterpene O-naphthoquinone of davidianone A, B, C and mansonone E, F, H, I from roots of U. davidiana were isolated (Kim et al., 1996). (+)-Catechin7-O-β.-Dapiofuranoside, lyoniside, 5’-methoxyisolariciresinol-9’- O-β.-D-xylopyranoside, rel-trans dihydrodehydroconiferyl alcohol 4-O-α-L-rhamnoside, icariside and ulmicine A-E from barks and roots of U. davidiana var. japonica were isolated (Bae et al., 2000), respectively.
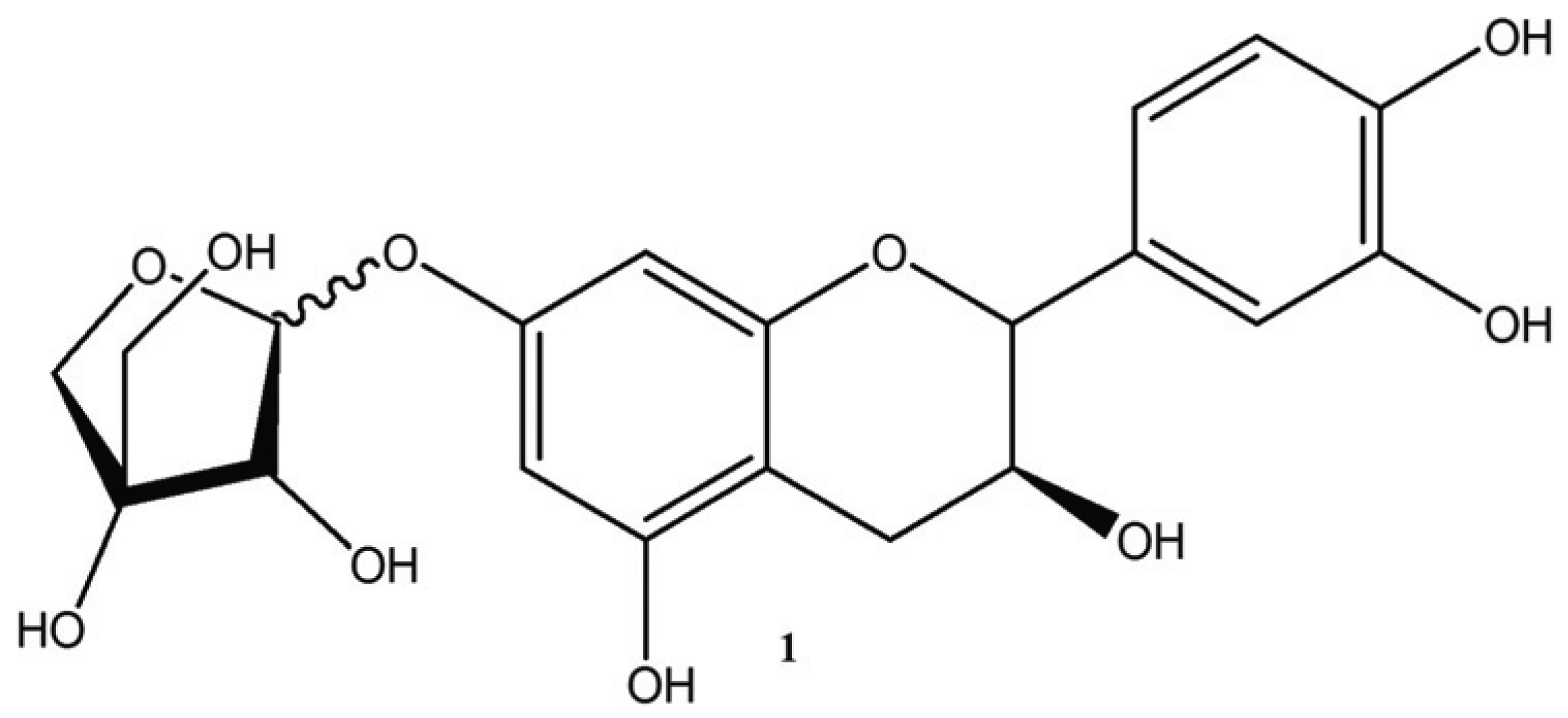
In this study, we isolated and identified catechin 7-O-beta-D apiofuranoside from the stems and barks of U. davidiana var. japonica for. suberosa. We also report the screening of catechin 7-O-beta-D apiofuranoside from other Ulmus species [U. davidiana Planchon (leaves, stems and barks), U. davidiana var. japonica (leaves, stems), U. parvifolia Jacq (stems and barks) and U. pumila Linne (stems)].
2. MATERIALS and METHODS
The stems and barks of U. davidiana var. japonica for. suberosa (bar code; PB2437.1) as well as MeOH extracts of U. pumila Linne (stems, bar code; PB2430.2), U. parvifolia Jacq (stems and barks, bar code; PB2427.1), U. davidiana var. japonica (stems, leaves, bar code; PB2436.6~7), and U. davidiana Planchon (leaves, stems and barks, bar code; PB2435.1~2) were purchased from the Korea Plant Extract Bank(Cheongju, Korea).
Thin layer chromatography (TLC) was carried out using a pre-coated silica gel 60 F254 plate (Merck, Darmstadt, Germany) with chloroform, methanol and water (70:30:4, volume ratio). The spots were detected under UV radiation (254 nm) and by spraying with FeCl3 and 10% H2SO4 followed by heating.
The components derived from the Ulmus species were identified by several instrumental analyses. Based on 1D nuclear magnetic resonance (NMR), 1H-(400MHz) and 13C-(100MHz) NMR experiments were recorded with FT-NMR Spectrometer 400 MHz, AVANCE III HD 400 (Bruker Corporation, Billerica, Massachusetts, USA) at Chonnam National University. Also, a high-resolution TOF mass spectrometry was conducted with JMS-T200GC (JEOL, Tokyo, Japan) at the National Center for Inter-University Research Facilities on Chonnam National University.
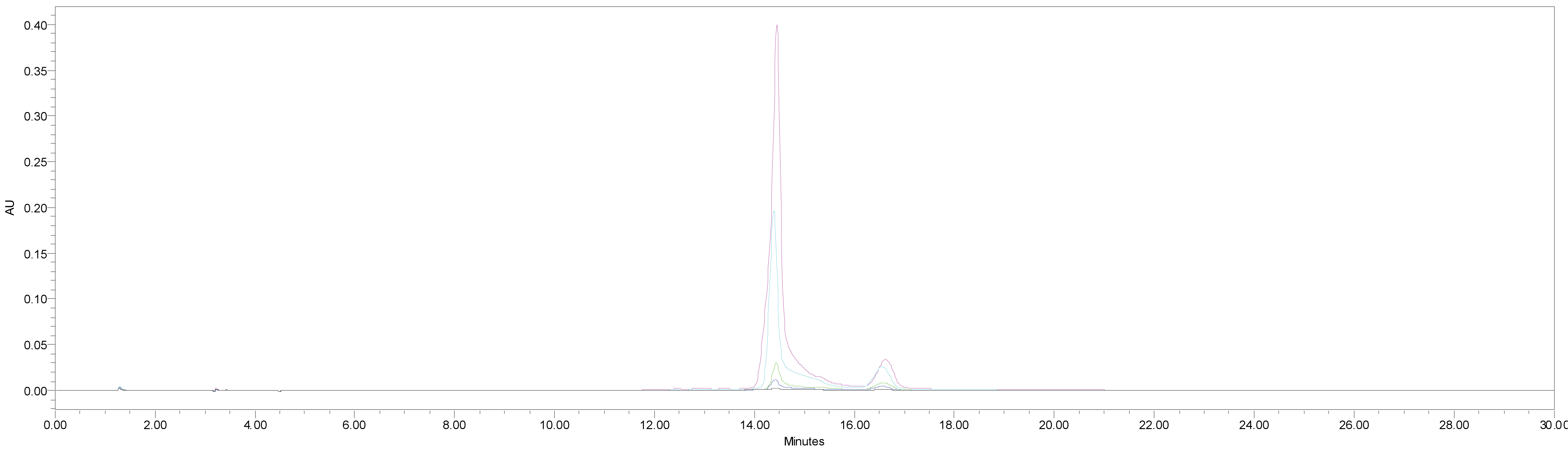
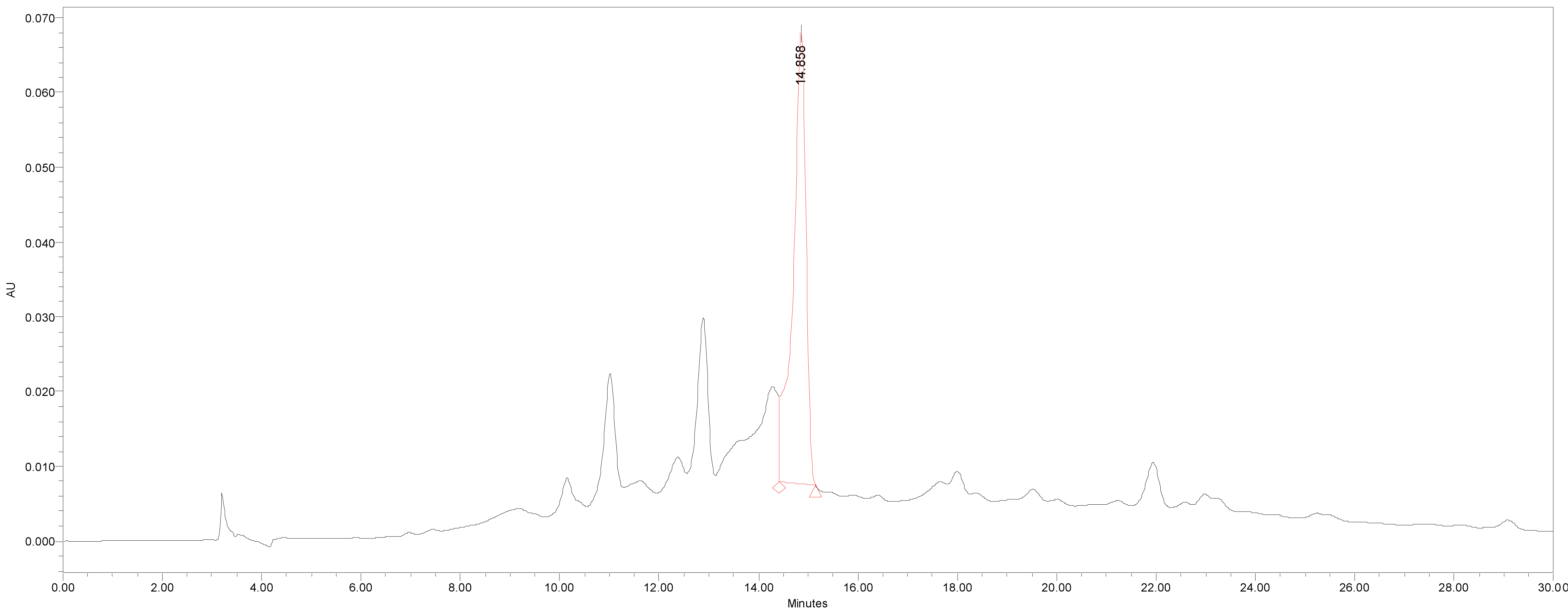
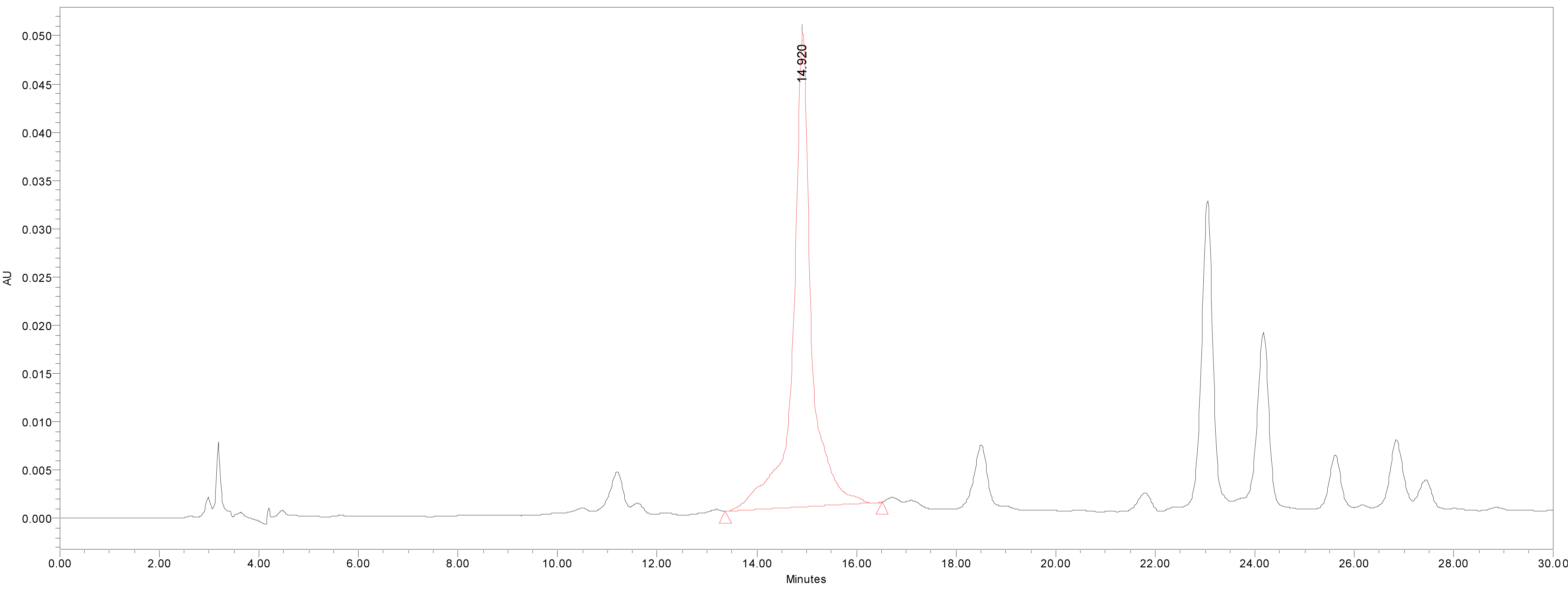
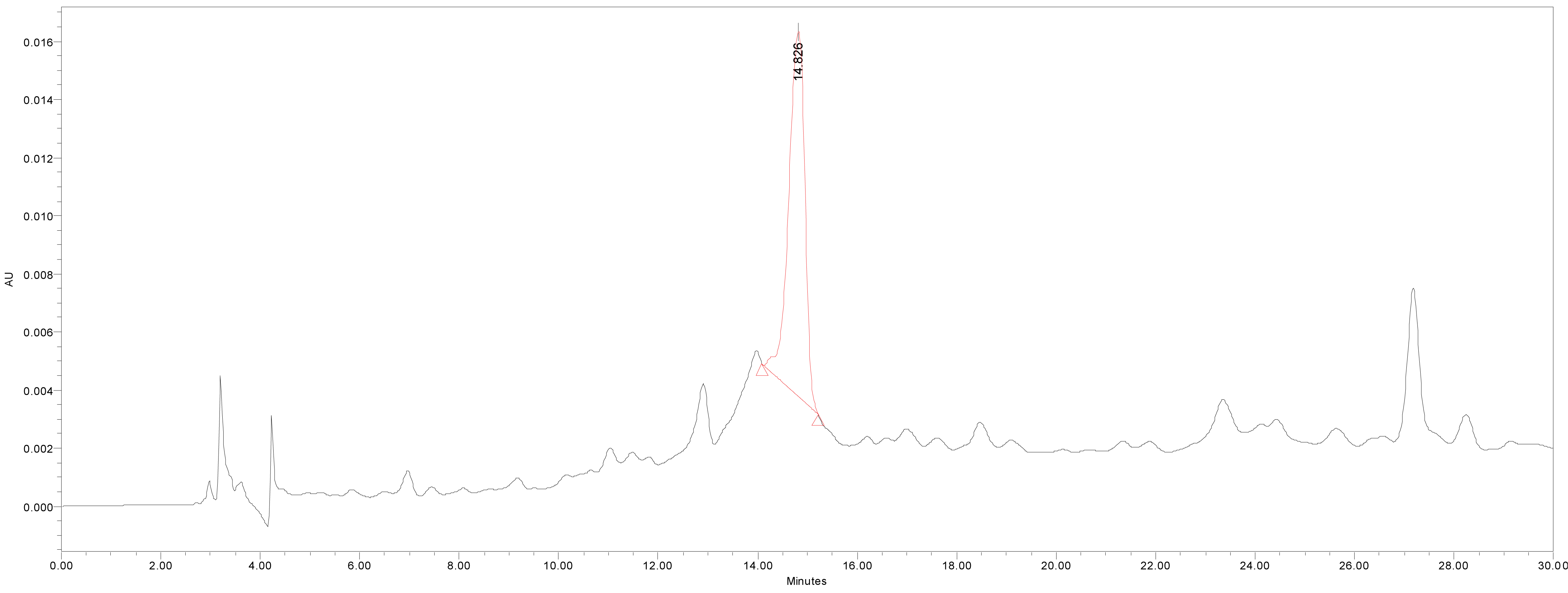
HPLC was used for the quantitative analysis of catechin 7-O-beta-D apiofuranoside. A Waters 2695 separation module (Milford, MA, USA) equipped with a vacuum degasser, a binary pump, a 2487 dual λ absorbance detector and column compartment, was used to separate 1 on a Phenomenex KJ0-4282 guard column and SkyPakC18 column (4.6 × 250 mm, 5 ìm particle) with a linear gradient [D.W. : MeOH : P2HO4 (940 : 50 : 1)] : [MeOH : P2HO4 (990 : 1)] = 100 : 0 to 0 : 100 for 30 min. The column temperature was maintained at room temperature. The flow rate was 1.0 mL/min. The injection volume of each sample was 20 μL. The system was monitored at 280 nm (λmax of catechin 7-O-beta-D apiofuranoside) eluting at14.44 ± 0.14 min, and catechin 7-O-β-D apiofuranoside was detected in the extracts of the stems and barks of U. davidiana var. japonica for. suberosa, as well as the extracts of U. davidiana Planchon (leaves, stems and barks), U. davidiana var. japonica (leaves, stems), U. parvifolia Jacq (stems and barks) and U. pumila Linne (stems).
3. RESULTS and DISCUSSION
Brown amorphous powder, high-resolution TOF MS m/z: 422.17511 [M]+; 1H-NMR (400 MHz, DMSO-d6 +D2O): 6.74 (H-2’, 1H, d, J = 2.0 Hz), 6.69 (H-5’, 1H, d, J = 8.4 Hz), 6.59 (H-6’, 1H, dd J = 2.0, 8.4 Hz), 6.09 (H-8, 1H, d, J = 2.4 Hz), 5.90 (H-6, 1H, J = 2.4 Hz), 5.33 (H-1’’, 1H, d, J = 4.0 Hz), 4.55 (H-2, 1H, d, J = 7.2Hz), 4.03 (H-2’’, 1H, d, J = 4.0 Hz), 4.00 (H-4a’’, 1H, d, J = 9.6Hz), 3.89 (H-3, 1H, m), 3.68 (H-4b’’, 1H, d, J = 9.6 Hz), 3.45 (H-5’’, 2H, m), 2.65 (H-4a, 1H, dd, J = 4.8, 16.0Hz), 2.40 (H-4b, 1H, dd, J = 8.0, 16.0z); 13C-NMR (100 MHz, DMSO-d6+D2O): δ 156.7 (C-7), 156.5 (C-5), 155.7 (C-9), 145.2 (C-4’), 145.1 (C-3’), 130.8 (C-1’), 118.8 (C-6’), 115.6 (C-5’), 114.7 (C-2’), 107.3 (C-1’’), 102.1 (C-10), 96.0 (C-8), 95.3 (C-6), 81.4 (C-2), 78.9 (C-3’’), 76.3 (C-2’’), 74.3 (C-4’’), 66.3 (C-3), 62.4 (C-5’’), 27.8 (C-4).
Compound catechin 7-O-beta-D apiofuranoside was brown amorphous powder. The spot was detected under UV radiation at 254 nm, the black spot was detected by spraying with FeCl3 and dark brown and orange spots was detected by spraying 10 % H2SO4 and anisaldehyde-H2SO4 followed by heating, respectively.
An aromatic ABX-spin system, the presence of meta-coupled aromatic signal [δ 6.74 (1H, d, J=2.0 Hz, H-2’)], ortho-coupled aromatic signal [δ 6.69 (1H, d, J=8.4 Hz, H-5’)] and ortho-meta-coupled aromatic signal [δ 6.59 (1H, dd, J=2.0, 8.4 Hz, H-6’)] was showed in 1H-NMR spectrum. The two carbon signals of C-3’ and 4’ were observed in downfield (δ 145.1, 145.2) compared with the peaks of C-2’, 5’ and 6’ (δ 114.7, 115.6 and 118.8) in 13C-NMR spectrum. These meant B-ring was pyrocatechol moiety which was substituted by hydroxyl group in C-3’ and 4’. The signals of two more aromatic protons, which were meta-coupled ones [δ 5.90 (1H, d, J=2.4 Hz, H-6), δ 6.09 (1H, d, J=2.4 Hz, H-8)] were observed in 1H-NMR spectrum, and the three carbon signals of C-5, C-7 and C-9, (δ 155.7, 156.5 and 156.7) were deshielded rather than C-6, 8 and 10 (δ 95.3, 96.0 and 102.1) in 13C-NMR spectrum.
These implied A-ring was related to phloroglucinol moiety which was substituted by hydroxyl group in C-5, 7 and 9. However the five carbon signals of 62.4 (api-5), 74.3 (api-4), 76.3 (api-2), 78.9 (api-3), 107.3 (api-1) indicate sugar existence in 13C-NMR spectrum. These signals were indicative of a D-apiofuranoside (Shengjun et al., 2000). The linkage of this sugar at C-7 was established by comparing the spectral data with the values reported in the literature (Na et al., 2002). Moreover, the proton signals of H-2 [δ 4.55 (1H, d, J=7.2 Hz)], H-3 [δ 3.89 (1H, m), H-4eq [δ 2.65 (1H, dd, J=4.8, 16.0 Hz)] and H-4ax [δ 2.40 (1H, dd, J=8.0, 16.0Hz)] were observed in 1H-NMR and the carbon signals in 13C-NMR spectrum of C-2, 3 and 4 (δ 81.4, 66.3 and 27.8) indicated flavan-3ol moiety. Especially, the large coupling constant of H-2 (J=7.2 Hz) in 1H-NMR and carbon signals of C-2 at δ 81.4 in 13C-NMR suggested a 2,3-trans-configuration.(Fig. 1, 2)
Thus, compound 1 was identified as catechin 7-O-β-D apiofuranoside based on the spectral data compared with the valuesreported in the previous studies (Jung et al., 2007; Bae et al., 2000; Shengjun et al., 2000; Na et al., 2002).
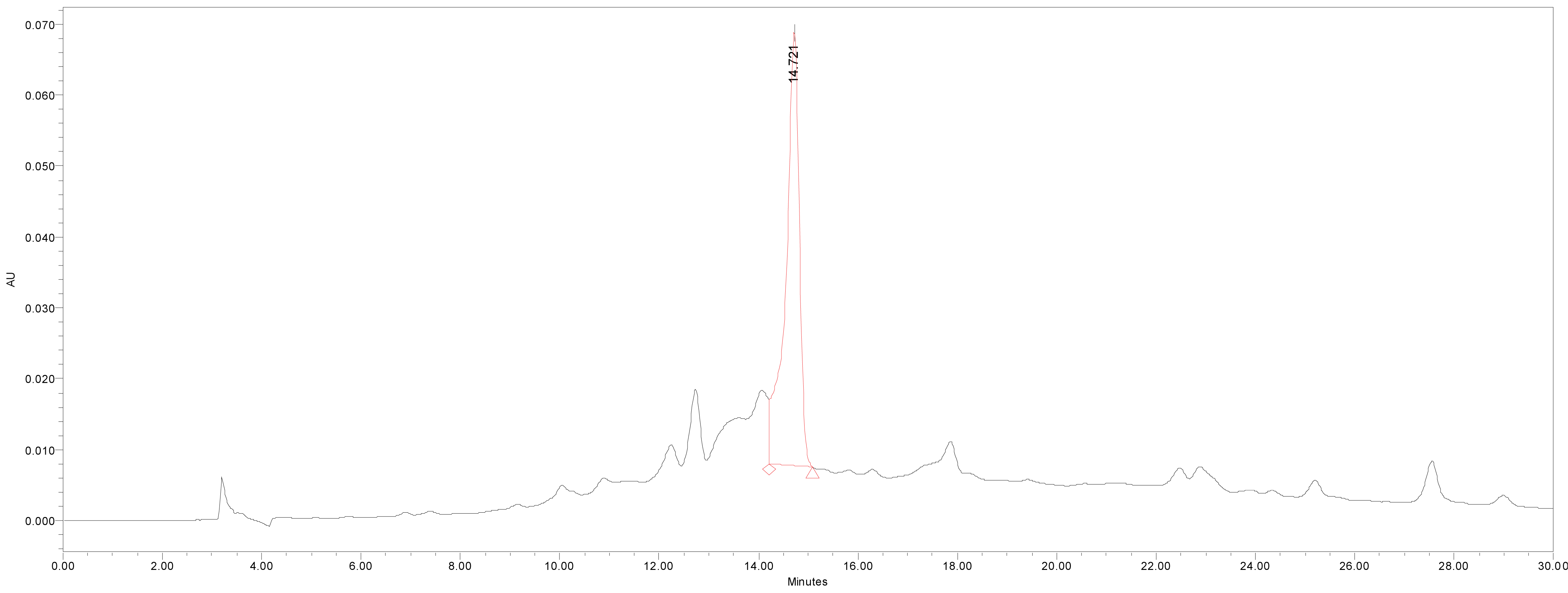
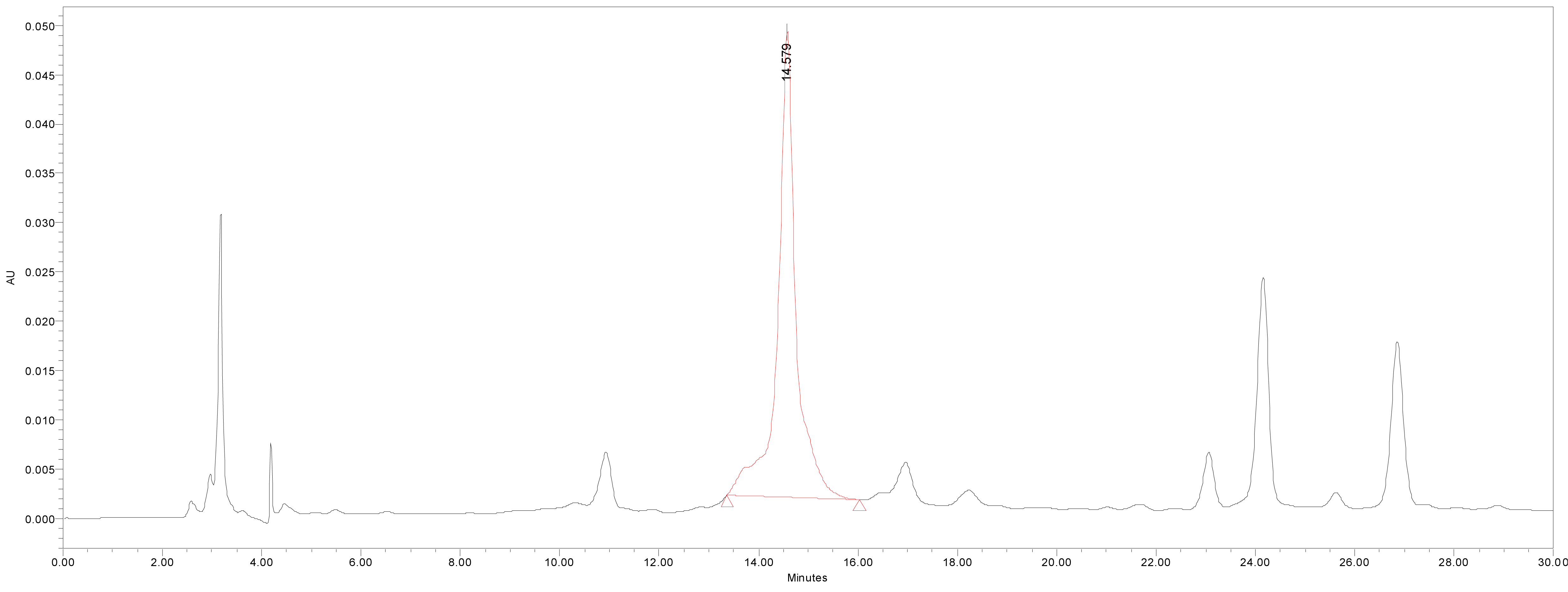
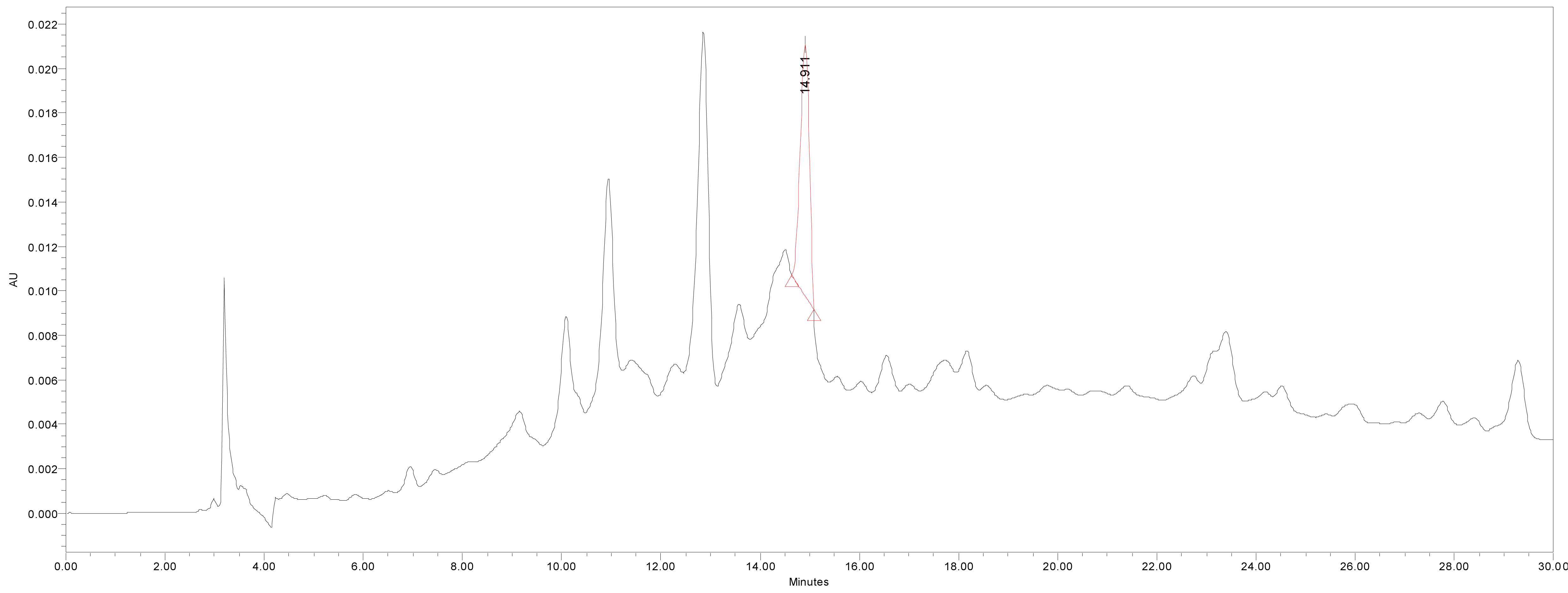
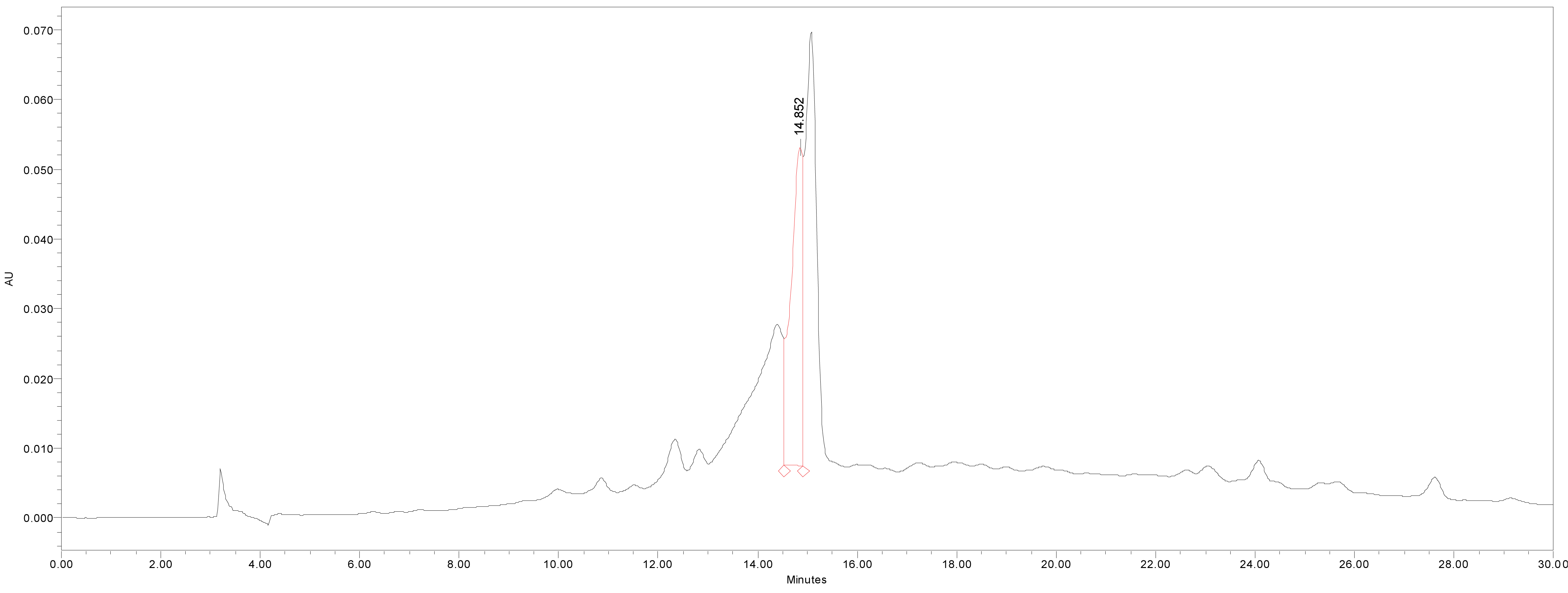
We quantified catechin 7-O-beta-D apiofuranoside from the stem and bark extracts of U. davidiana var. japonica for. Suberosa [172.35 ± 0.158 ppm (μg/mL), 17.24 ± 0.016%] (Fig. 3), the leaf extracts of U. davidiana Planchon [191.38 ± 0.219 ppm (μg/mL), 19.14 ± 0.022%] (Fig. 4), the stem and bark extracts of U. davidiana Planchon [177.84 ± 0.209 ppm (μg/mL), 17.78 ± 0.021%] (Fig. 5), the leaf extract of U. davidiana var. japonica [176.11 ± 0.155 ppm (μg/mL), 17.61 ± 0.015%] (Fig. 6), the stem extract of U. davidiana var. japonica [41.27 ± 0.191 ppm (μg/mL), 4.13 ± 0.019%] (Fig. 7), the stem and bark extracts of U. parvifolia Jacq [150.88 ± 0.205 ppm (μg/mL), 15.09 ± 0.020%] (Fig. 8) and the stem extract of U. pumila Linne [61.69 ± 0.178 ppm (μg/mL), 6.17 ± 0.018%] (Fig. 9), using a calibration equation (y = 6857.3x – 42331; R2 = 0.9985). According to the results of the content analysis experiment using HPLC, there was no significant difference in the content of the indicator substance in the other samples except for the two samples (the stem extract of U. davidiana var. japonica and the stem extract of U. pumila Linne), but the two samples with a significantly lower level were important experimental results when evaluating the value of future use of raw materials. It is expected to be used as important basic data in future resource utilization.
4. CONCLUSION
This is the first report of the isolation and identification of catechin 7-O-beta-D apiofuranosidefrom U. davidiana var. japonica for. suberosa., U. parvifolia Jacq and U. davidiana Planchon.Since the initial isolation of catechin 7-O-beta-D apiofuranoside from U. Americana (Doskotch et al., 1973), it has been found to be distributed among U. davidiana var. japonica (Jung et al., 2007) and U. macrocarpa Hance (Kwon et al., 2011).
Based on the above results, it is expected that it will be used as an important research result in searching for new natural materials that can be given high added value.