1. INTRODUCTION
In 2019, over 85% of wood was imported to Korea, of which timber imports accounted for around 10%. About 75% of the imported timber is P. radiata which came from New Zealand with an import volume of approximately 2 million m3 (KFS, 2020). P. radiata wood is currently used in various fields such as construction, packaging, furniture and joinery, as well as in the production of plywood and medium-density fiberboard (Bayne, 2015). P. radiata bark accounts for 10 – 12% of total timber (Murphy and Cown, 2015). Taking this into consideration, about 200,000 m3 of bark per year is discharged from wood and its related industries in Korea. The bark is currently used as fuel, compost, and landscaping products (Murphy and Cown, 2015).
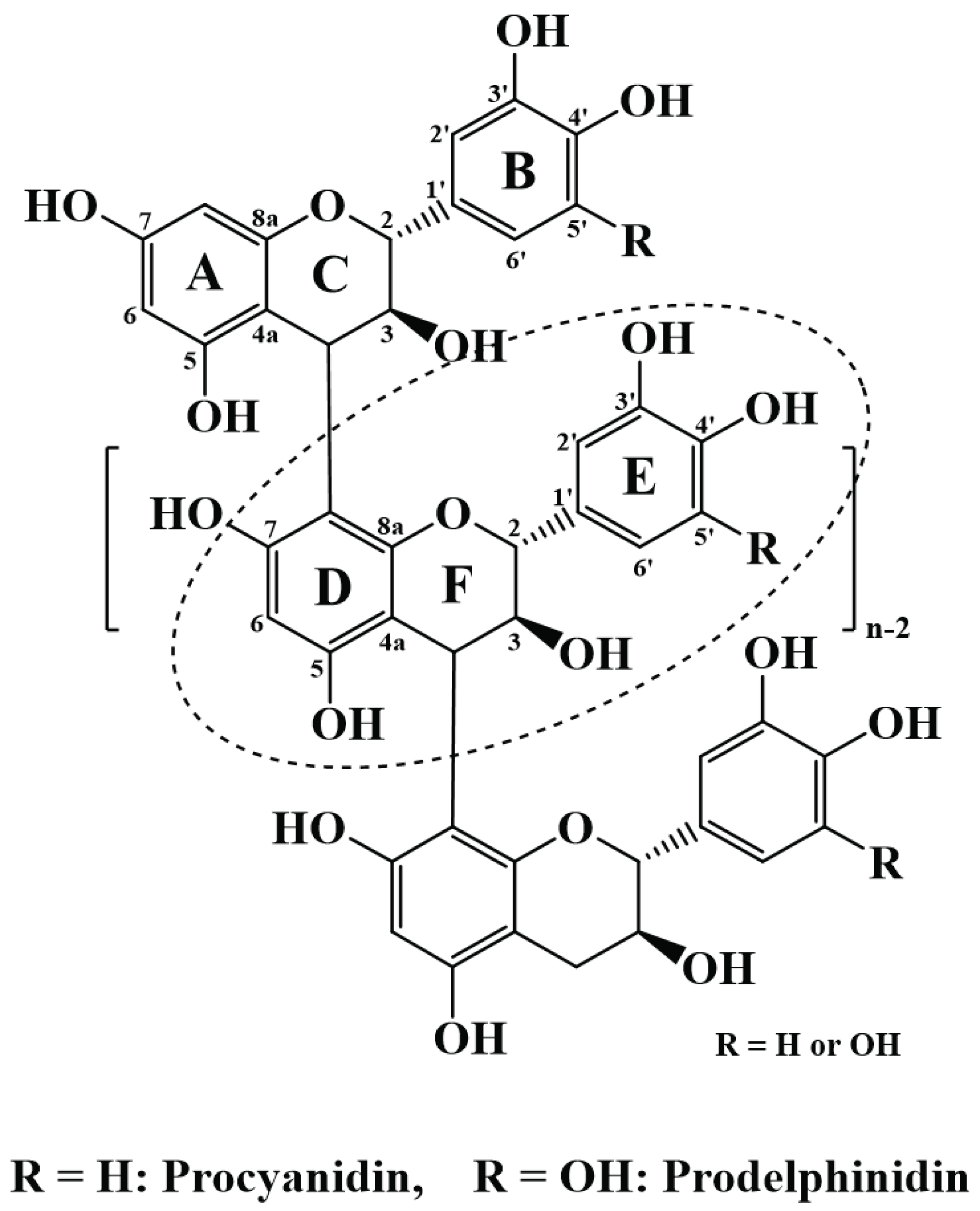
The bark of P. radiata is a rich source of proanthocyanidins (PAs, Fig. 1) (Ku et al., 2011), a potent antioxidant (Prior and Gu, 2005; Ku and Mun, 2008; Ku et al., 2011; Mun, 2014). Studies of pine bark extracts have been conducted and the extracts have shown anti-hepatotoxic, anticarcinogenic, antioxidant, and antiinflammatory effects (Rohdewald, 2002; Kim and Park, 2004; Ku and Mun, 2008). For that reason, pine bark extracts are sold worldwide as health supplements and cosmetic additives under the name of Pycnogenol®, Enzogenol®, and Pinoradiol® (Packer et al., 1999; Ghosh and Mukherjee, 2009; Choi et al., 2018). PA-rich extracts from P. radiata bark have also shown the potential as a termite deterrent (Mun and Nicholas, 2017) and as an adhesive for particle board production (Pizzi, 1982).
Bark extraction can be conducted in alkaline, cold water, hot water, organic solvents, and neutral conditions. The bark of P. radiata is easily extracted in highly alkaline conditions, but severe chemical modifications of the polyphenols occur and the solubility of the alkaline extracts are greatly decreased due to the formation of phlobaphenes by the rearrangement of bark polyphenols such as PA (Sealy-Fisher and Pizzi, 1992; Jeong and Mun, 2018). Hot water extraction is commonly used because PAs, which are a major component in most pine barks, have higher solubility in hot water than cold. Furthermore, hot water extraction is environmentally safer than using organic solvent extraction. However, when hot water extracts (HWE) are cooled down, precipitation occurs. In addition, after drying, when powdered extracts are dissolved in water again, many insoluble portions generated by the association between PA molecules make the use of these products very difficult (Mun and Lim, 2008).
On the other hand, Mun and Lim reported that when P. radiata bark is extracted using a weak alkali, such as Na2CO3 and NaHCO3, and the final extraction pH becomes neutral, extract yields are higher than with hot water. The extracts do not precipitate even after cooling and also dissolves well after drying. They also reported that the extracts retain strong antioxidant properties similar to pure PA (Mun and Lim, 2008). Since the weak alkaline extraction of P. radiata bark gave higher extract yields than HWE, this extraction would be economical. In addition, the extracts had high antioxidant activity which would be beneficial to health supplement, functional cosmetic, and natural dyestuff industries. However, in the previous study, the chemical characterization of NE was performed partially, thus, a more detailed analysis and information on NE should be needed for further utilization of P. radiata bark. Therefore, in this study, P. radiata bark was extracted with NaHCO3 aqueous solution to prepare NE and the chemical characteristics of NE were investigated in comparison with pure PA prepared from the same pine bark.
2. MATERIALS and METHODS
The bark used in the experiment was kindly provided by Baeksan Wood Co., Ltd. (Gunsan-si, South Korea) and the age of the P. radiata wood was 70 - 80 years old. After removing the scales and inner bark, the remainder was crushed with a hammer and then ground with a blender (Primaire, Hanil, South Korea). The ground bark was sieved through 40 mesh. The powder that passed through the mesh was stored in a zipper bag at room temperature and was used as the material for this study.
Sodium bicarbonate (NaHCO3, 99.5%), acetic anhydride (93.0%), tetrahydrofuran (THF, 99.9%), and methanol (MeOH, 99.9%) were purchased from Duksan Pure Chemical Co., Ltd. (South Korea), pyridine (99.5%) from Kanto Chemical (Japan), 2-diphenyl-1- picrylhydrazyl (DPPH) radical from Sigma- Aldrich (Germany), deuterated acetone (acetone-d6) and deuterium oxide (D2O) from Merck KGaA (Germany).
The ash, cold water, hot water, 1% NaOH, alcohol- benzene extract, and total lignin content of the P. radiata bark were measured in accordance with TAPPI test methods (T 204, 2007; T 207, 2008; T 211, 2002; T 212, 2002; T 222, 2006). However, the lignin content of the pine bark obtained via Klason method was higher than the accepted values because of the presence of polyphenols. Thus, in this study, the bark polyphenols were extracted with 1% NaOH, and thereafter the polyphenols-corrected lignin value was determined using the residues following 1% NaOH extraction.
A 100 g (o.d.) of the bark powder (40 mesh passed) and 500 mL of 0.8% NaHCO3 aqueous solution were added to a 1 L round bottom flask. The flask was fitted to a condenser and then placed in a PEG #400 bath preset at 110°C. The extraction was carried out for 1 h from the start of boiling with occasional shaking. After the extraction, the bark slurry was filtered through a 25G3 glass filter (Pyrex, UK), and the residue was washed with 500 mL of hot distilled- deionized water (DI-water). After washing, the glass filter was dried in a 105°C convection oven (FO-600M, Jeio Tech, South Korea) for 48 h. The yield of NE was then calculated as follows (1):
where, BP is bark powder (g), o.d. is oven dried weight, and RE is residue after extraction.
After cooling NE, an aliquot was taken into a 30 mL conical beaker and the pH was measured by pH meter (CyberScan pH 1500, Thermo, USA). The NE was equally transferred into four 1 L eggplant flasks. The extracts in the flasks were frozen by carefully rotating the flasks at -35°C in a low temperature circulation bath (RBC-30, Jeio Tech, South Korea). The flasks were lyophilized for 24 h in a freeze dryer (FDU-540, Eyela, Japan). The dried extracts in the flasks were transferred into a 950 mL plastic bottle and then vacuum dried for 80 h at room temperature in the presence of P2O5.
A 500 mg of the thoroughly dried NE and 10 mL of anhydrous pyridine were added into a 50 mL Erlenmeyer flask. The flask was sonicated for 90 s to disperse the mixture. Afterwards, 10 mL of acetic anhydride was added and then stirred with a magnetic stirrer (SR-306, Advantec Toyokaisha, Japan) for 48 h at room temperature. After 48 h, 175 g of crushed ice and 200 g of DI-water were added into a 500 mL beaker and was stirred vigorously with a magnetic stirrer (RCN-7, Eyela, Japan). The acetylated sample was sprayed into the beaker by using a tapered pipette and allowed to stir for 1 h at room temperature. The acetylated precipitates were collected by filtration using a nylon 66 membrane filter (47 mm diameter, 0.45 μm, Alltech, USA). The precipitates on the filter were washed with 300 mL of DI-water. The filter was placed in an aluminum dish and then vacuum dried for 24 h in the presence of P2O5.
A 2 mg of the acetylated NE (Ac-NE) and 1 mL of THF were added into a 10 mL conical beaker. The beaker was sonicated for 10 s for complete dissolution and then filtered through a 0.45 μm nylon syringe filter (Phenomenex, Torrance, CA, USA). The filtrate was transferred into a 2 mL vial and subjected to GPC. The acetylated pure PA (Ac-pure PA) prepared from P. radiata bark was used in this experiment as a sample for comparison. The GPC was conducted under the conditions shown in Table 1. The molecular weight (MW) of the samples was calculated from the standard curves prepared with phenol and polystyrene standards.
The analysis was performed using the ATR method at 400 – 4000 cm-1 with an FT-IR spectrophotometer (Frontier, Perkin Elmer, USA) in the Center for University- Wide Research Facilities (CURF) at Jeonbuk National University. The measurement was conducted under conditions of 20°C with 44% relative humidity.
A 100 mg of the Ac-NE, 0.4 mL of D2O, and 0.2 mL of acetone-d6 were added into a 10 mL conical beaker. The beaker was sonicated for 1 min to completely dissolve the sample. The solution was passed through a filter formed by placing a fine glass wool for GC inside a Pasteur pipette and the filtrate was immediately transferred into an NMR tube. The conical beaker was washed with additional D2O and acetone-d6 in a 2 : 1 (v/v) ratio. The solution was transferred onto the filter to dissolve the remaining part in the filter and was received into the NMR tube again. The measurement was conducted with a 600 MHz FT-NMR spectrometer (JNM-ECA600, JEOL Ltd., Japan) in the CURF at Jeonbuk National University.
Both Ac-pure PA and NE samples after GPC analysis were also used for MALDI TOF MS analysis. A 10 mg of NaCl and 10 mg of 2,5-dihydroxybenzoic acid (DHB) matrix were dissolved in 1 mL of 50% acrylonitrile containing 0.1% trifluoroacetic acid. The sample solution, NaCl solution, and DHB were mixed in the ratio of 1 : 1 : 1, and a 1 μL aliquot sample was used for the analysis. The mixture was placed on a MALDI target, and the solvent was air dried. The measurement was conducted with MALDI TOF MS (Applied Biosystems VOYAGER-STR MALDI TOF MS, USA) at Korea Basic Science Institute (Jeonju).
The antioxidant activity of the NE was determined by DPPH free radical scavenging assay. The DPPH solution was prepared by dissolving 1.78 mg of DPPH in 30 mL MeOH in a 50 mL Erlenmeyer flask, and the flask was covered with aluminum foil. A 5 mg of NE and 25 mL MeOH were added to another 50 mL Erlenmeyer flask. The flask was sonicated for 1 min and then allowed to stand for 30 min at room temperature. This solution was considered the stock solution. Various concentrations of solutions were made by diluting the stock solution with MeOH. The pure PA sample was prepared with the same method for comparison. Triplicate sets of 150 μL diluted solutions were placed in a 96-well plate (Corning Incorporated, USA) using a multi- pipette (Eppendorf, Germany) and 150 μL of DPPH solutions were added. The blank consisted of 300 μL of MeOH, and the control was 150 μL of DPPH and 150 μL of MeOH. The plate was left in a microplate reader (ELx808, Bio- Tek, USA) set at 28°C for 30 min. The measurement was conducted at an absorbance of 515 nm. DPPH free radical scavenging activity was calculated as follows (2):
where, A0 is absorbance of the control and A1 is absorbance of the sample.
3. RESULTS and DISCUSSION
Analysis of ash, extracts, lignin, and polyphenol content was performed to determine the chemical composition of P. radiata bark and the results are shown in Table 2. The content of ash, cold-water, hot-water, and alcohol-benzene extracts was 0.42%, 20.45%, 32.81%, and 12.69%, respectively. The yield of the alkaline extract was more than 70%, significantly higher than that of cold-water, hot-water, and organic solvent extracts as reported by Mun and Lim (2008). In contrast, the majority of the polyphenols in P. radiata were extracted by 1% NaOH (Jeong and Mun, 2018). Hence, the 1% NaOH extracts can be used as an estimate of the total polyphenolic content. From the calculation, the total polyphenol content was 53.94% corresponding to around 74% of the 1% NaOH extract.
When P. radiata bark was extracted with 0.8% NaHCO3 aqueous solution, the yield of the bark extracts and their pH were 44% and 6.66, respectively. As shown in Table 2, the yield of HWE from P. radiata bark was around 33%. However, when this bark was extracted with weak alkali to attain neutral pH, the yield increased by more than 10%. This was thought to be due to the increased solubility of bark polyphenols such as PAs in weakly alkaline conditions. The chemical structures and composition of NE were examined through GPC, FT-IR, 13C NMR, and MALDI TOF MS analyses.
GPC of Ac-NE and Ac-pure PA was performed to determine the number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity (Mw/Mn). Fig. 2 shows the MW distribution of Ac-pure PA and Ac-NE. The high and low MW fractions of NE were more widely distributed than those of pure PA. In addition, the peak MW of NE was higher than pure PA.
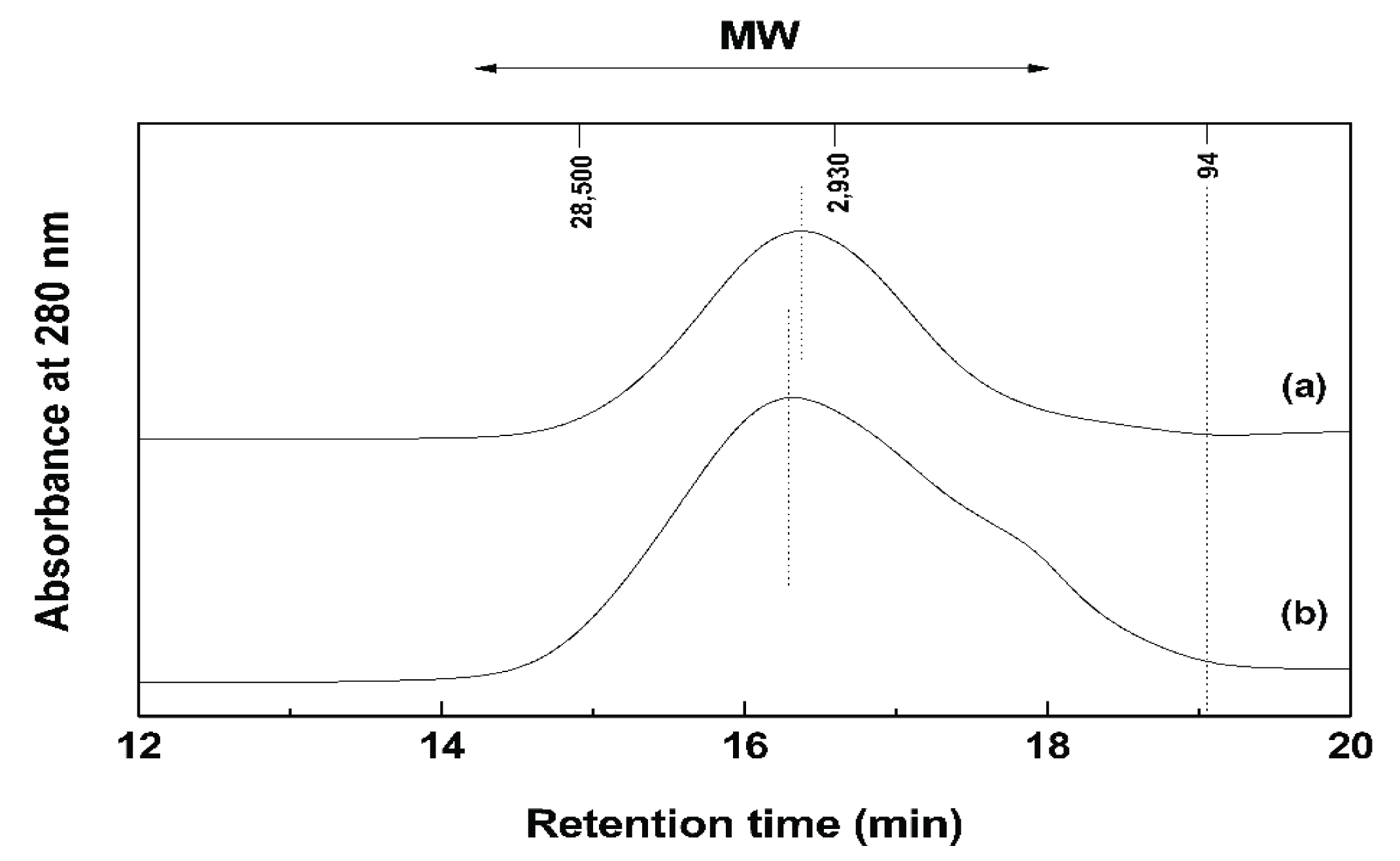
Table 3 shows the values for Mn, Mw, and polydispersity of Ac-pure PA and Ac-NE. For Ac-NE, the Mw was about 600 Da lower and the n was 1/6 that of Ac-pure PA because the MW of Ac-NE was widely distributed from high to low MW fractions as shown in Fig. 2. Therefore, it was clear that a broad range of UV-absorbing materials of different molecular sizes were extracted from the bark since the polydispersity of NE was 6 times higher than that of pure PA.
| Sample | Mw | Mn | Mw/Mn |
|---|---|---|---|
| Ac-pure PA | 5897 | 1854 | 3.2 |
| Ac-NE | 5289 | 286 | 18.5 |
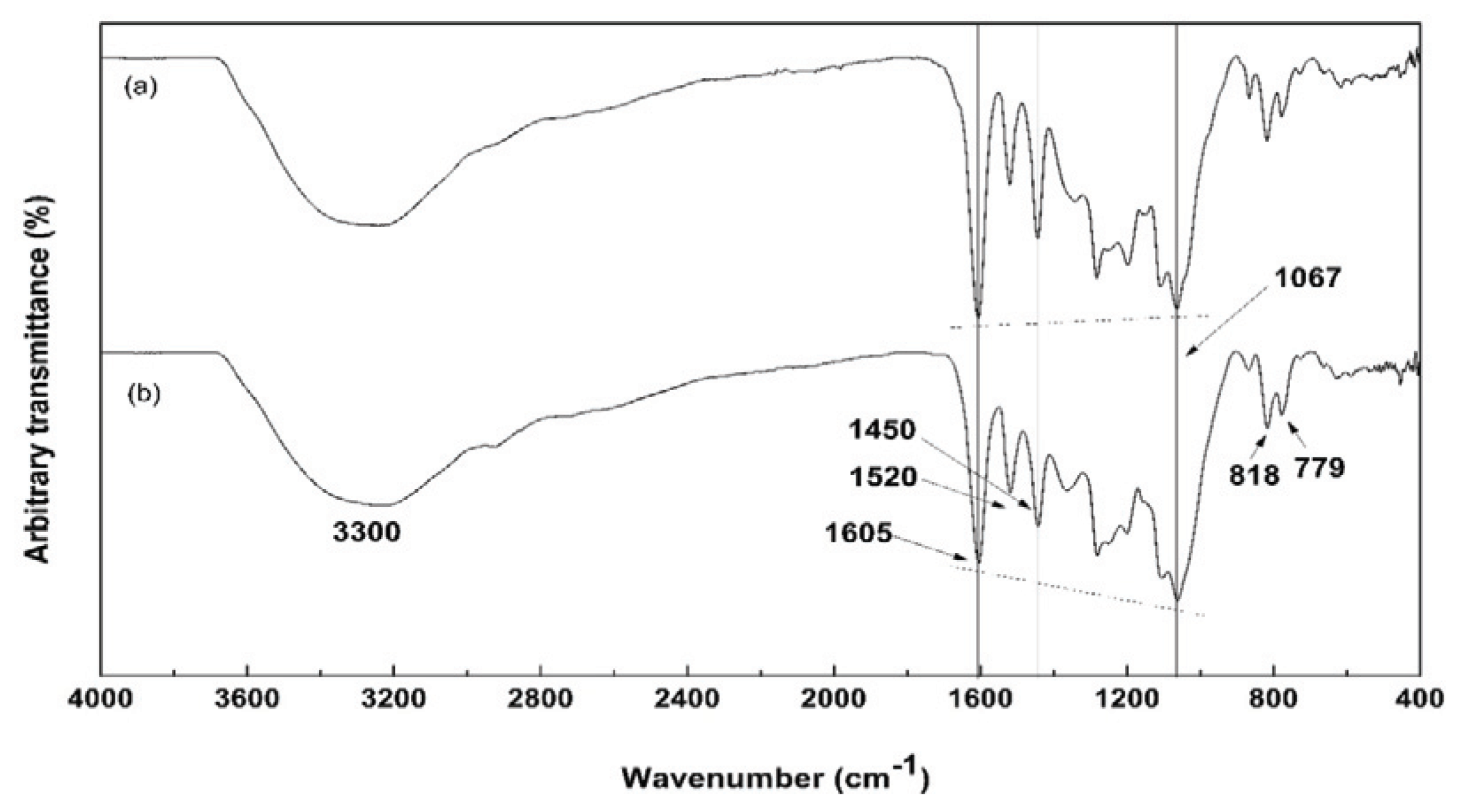
The FT-IR analysis was performed to determine the functional groups in NE. Fig. 3 shows the FT-IR spectra for pure PA and NE. The overall FT-IR spectrum pattern of NE was similar to that of the pure PA prepared from the same pine bark. The characteristic bands of the aromatic rings appeared at 1605, 1520, and 1450 cm-1 with the strong and broad stretching bands of the -OH group of polymeric phenol at 3300 cm-1 in both spectra. Also, characteristic bands of typical polyflavonoids were present including symmetric stretching bands of ether groups (=C-O-C bonds) as shown at 1067cm-1 (Yazaki and Hillis, 1977). This confirmed the presence of PAs in NE. In addition, NE showed a single band at 1520 cm-1 and 779 cm-1 like pure PA. This suggested that NE was mainly composed of procyanidin (PC) structure of PAs.
The strength of the 1067 cm-1 absorption band in NE was higher than that of the pure PA. The strong C-O single-bond stretching vibrations were observed in the range from 1260 to 1000 cm-1. The spectrum of a primary alcohol, such as 1-hexanol, has its C-O absorption at 1058 cm-1, cyclohexanol and secondary alcohols had their absorption bands of 1070 cm-1 and 1060 cm-1, respectively (Pavia et al., 1996). Thus, it was predicted that the increased strength of 1067 cm-1 band in NE was mainly due to the presence of primary and secondary aliphatic alcohol’s and the C-O stretching absorption. Although pure PA also has an aliphatic -OH group at C3 as shown in Fig. 1, it was thought that NE, exhibiting increased strength of this absorption band, had compounds other than aliphatic -OH groups such as carbohydrates. In fact, it is known that there are small amounts of carbohydrates in P. radiata bark (Newman and Porter, 1992) some of which are probably solubilized during the weakly alkaline extraction.
13C NMR was performed to obtain more detailed information on the chemical structures and composition of NE. Fig. 4 shows the 13C NMR spectra of Ac-pure PA and NE. For both spectra, the peaks of PC units, 116 ppm (2'B, 5'B), 120 ppm (6'B), and 145 ppm (3'B, 4'B, 3'E, 4'E) were observed. Recently, the PC/PD (procyanidin/prodelphinidin) ratio of PAs was determined by the intensity ratio at 116/107 ppm (Kraus et al., 2003). According to this measurement, NE were composed of 90% PC and 10% PD units. This result indicated that the major component present in NE of P. radiata bark is PC, which was consistent with the FT-IR analysis. In addition, the signals between 60 ppm and 80 ppm originated from the peaks of sugars as expected from the FT-IR analysis, and the C2 and C3 signals overlapped with these sugar-related signals.
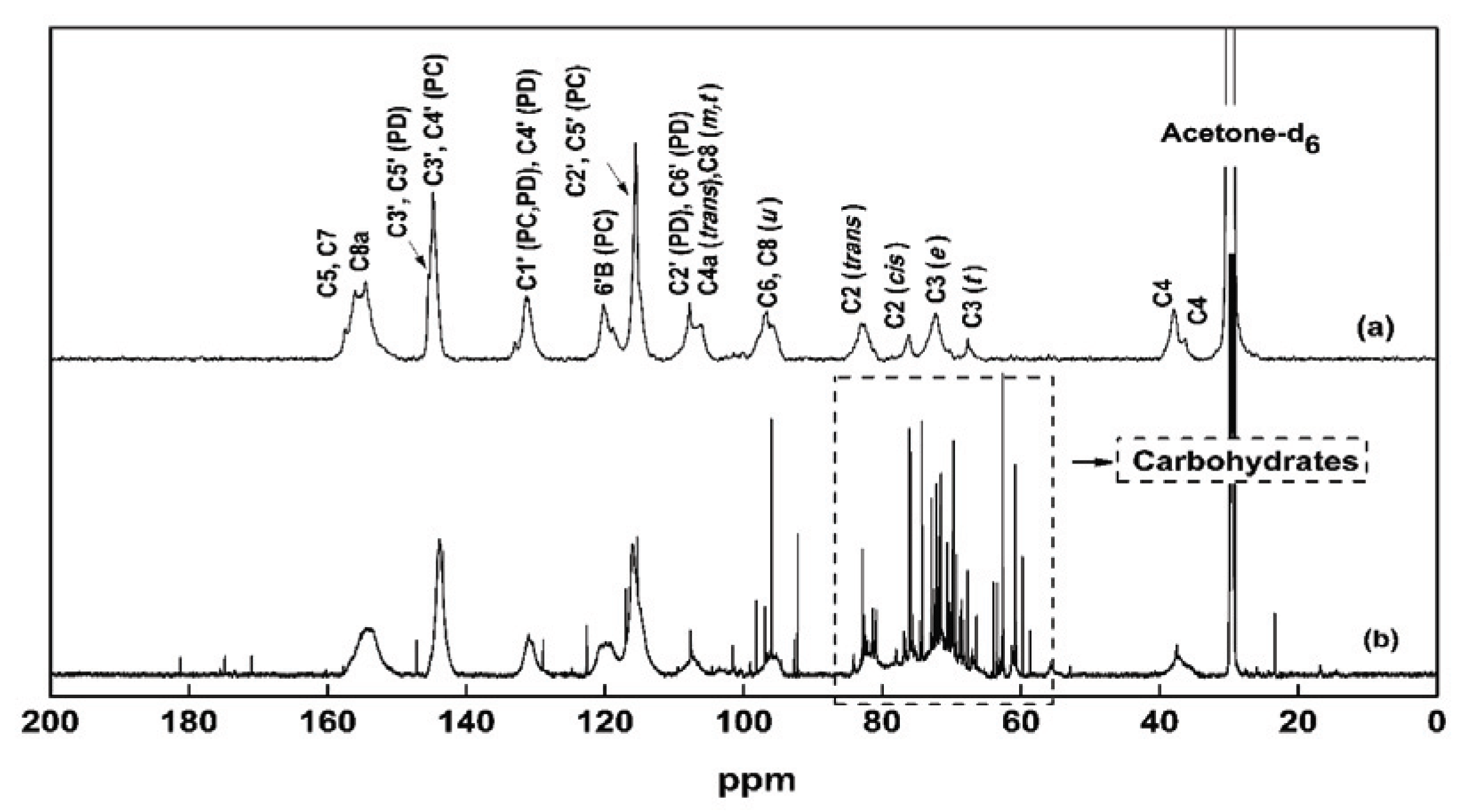
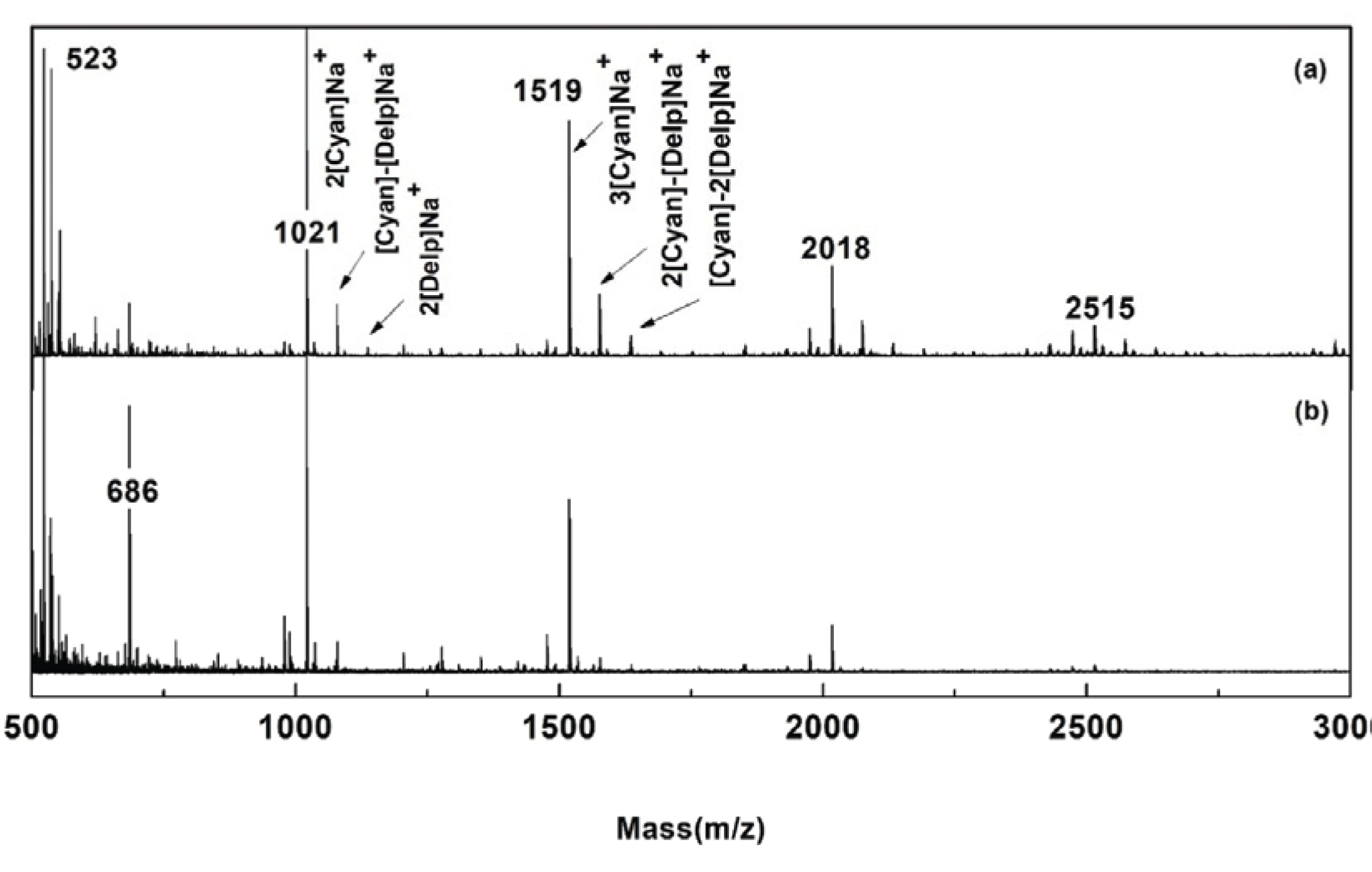
The FT-IR and 13C NMR spectra indicated that NE were mainly composed of PAs and contained small amounts of sugar. MALDI TOF MS analysis was performed to obtain more detailed information on the sub-structures and MW of NE repeating units. Fig. 5 shows the MALDI TOF MS spectra of Ac-pure PA and Ac-NE. Table 4 summarizes the calculated MWs of dimers, trimers, and oligomers present in the acetylated samples. The basic repeating unit in pure PA prepared from P. radiata bark is flavan-3-ol, which has total of 5 hydroxyl groups in the backbone. When all of these hydroxyl groups in the basic unit are substituted with acetyl groups and Na+ is added, the MW becomes 522 Da. In Fig. 5, the peak strengths at m/z ratios of 1021, 1519, 2018, and 2515 were very high. These peaks were correspondent to dimer, trimer, tetramer, and pentamer of the PC unit, respectively (Table 4). Peaks at 1079 and 1579 were also detected in NE. The peak at 1079 indicates the presence of one cyanidin unit and one dephinidin unit as shown in Table 4. The peak at 2079 indicates the presence of two PC units and one PD unit. The presence of PD unit in NE was expected based on the 13C NMR analysis. From MALDI TOF MS analysis, we concluded NE were mainly composed of low MW PAs of dimers and trimers. The peak at 686 only appeared in NE but a structure could not be assigned and further studies will be needed for this peak assignment.
From MALDI TOF MS analysis, it was confirmed that the majority of the compounds present in NE were PC with traces of PD. This result highly corresponded with the 13C NMR result. In MALDI TOF MS analysis, flight time varies depending on the temperature and the voltage. Also, the laser intensity fluctuations affect the signal intensity of analytes, resulting in overlapping peaks of compounds of similar MW (Vorm et al., 1994; He et al., 1995). In fact, the MW of PCs in this experiment was very similar to that of PCs isolated by Ku and Mun (Ku and Mun, 2007).
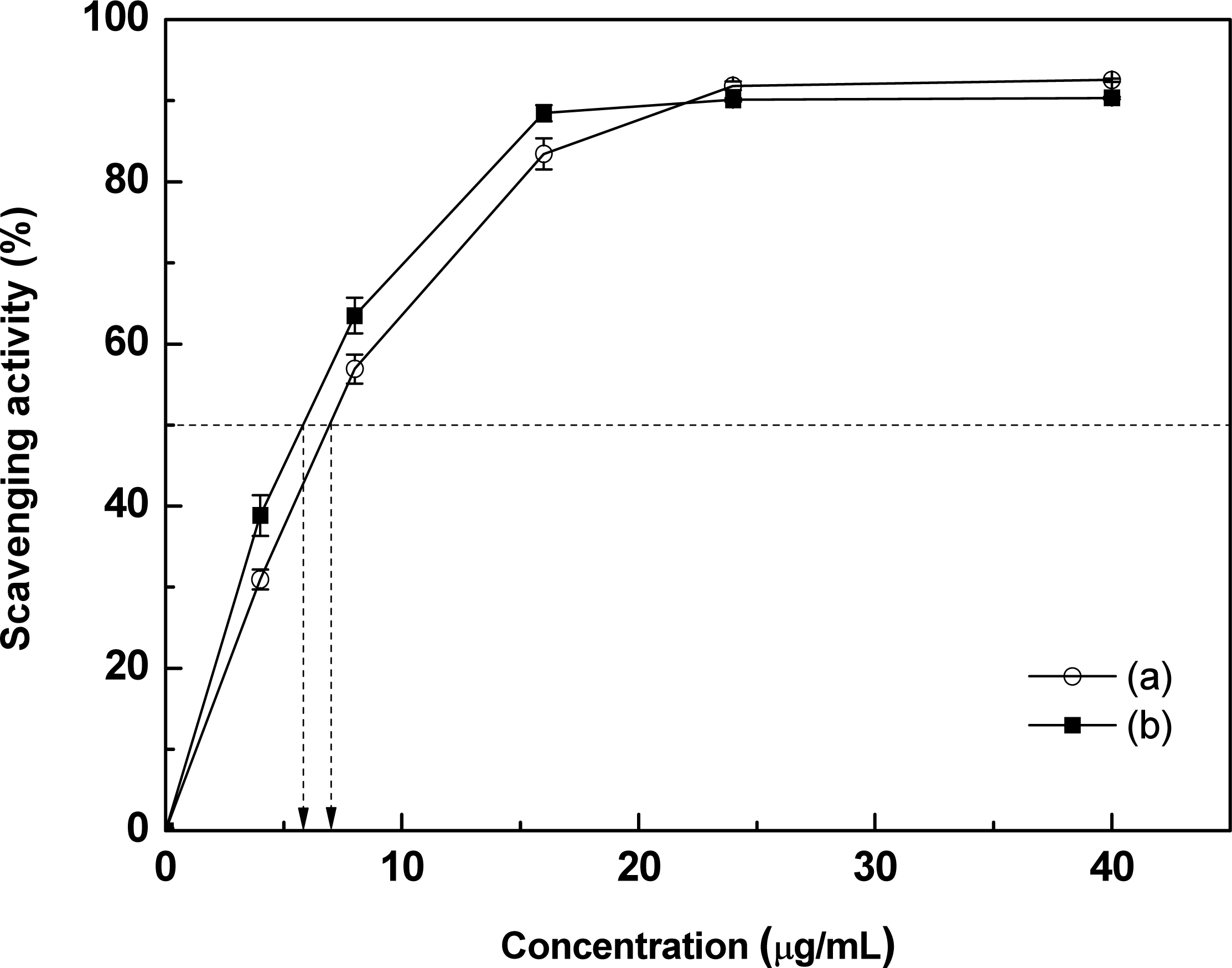
The antioxidant activity of NE was determined by DPPH free radical scavenging assay. Fig. 6 shows results for pure PA and NE. Both pure PA and NE showed an overall similar tendency in free radical scavenging activity, but it was thought that NE had slightly higher antioxidant activity than pure PA. Specifically, the concentration of DPPH free radical required to reach 50% scavenging activity was 5.9 μg/mL for NE but 7 μg/mL for pure PA. We speculated that NE exhibited a slightly higher activity than pure PA due to the presence of a component having a higher antioxidant activity in addition to the main PA component present in NE. This assay confirmed that NE, like pure PA, exhibited strong antioxidant activity even at extremely low concentrations.
4. CONCLUSION
When P. radiata bark was extracted with 0.8% NaHCO3 solution, the neutral extract (NE) with a pH of 6.66 was obtained in 44% yield, which accounts for about 60% of the 1% NaOH extract. The NE was characterized through various instrumental analyses. GPC analysis showed that NE had a wider MW distribution and lower Mw (5,300 Da) than pure PA. From the FT-IR, 13C NMR, and MALDI TOF MS analyses, it was confirmed that NE was mainly composed of PAs consisting of PC units and small amount of sugars as a minor component. NE exhibited a potent antioxidant activity similar to pure PA. Consequently, the weak alkaline extraction of P. radiata bark not only had a high extract yield, but also had high antioxidant activity and solubility in cold water, so the extract would be beneficial to the health supplement, functional cosmetic, and natural dyestuff industries in the future.