1. INTRODUCTION
Vitiligo is a common depigmenting skin disease, associated with certain autoimmune endocrinopathies, and autoantibodies to several antigens can be found in melanoma cells. Vitiligo is an acquired condition without other apparent presymptoms in the skin (Spritz, 2010). It was suggested that there were melanocytes but their melanin producing activity was inhibited. When melanocyte activity is affected, melanin is not synthesized in the melanosomes of melanocytes. In addition, the development of vitiligo is related to the rates of synthesis and decay of tyrosinase. As present, many studies have investigated the importance of tyrosinase in the regulation of racial pigmentation. Song et al. (1994) showed that tyrosinase was an enzyme important in melanin formation. The current treatment options for vitiligo include medication, surgery, and adjunctive therapies (those used along with surgical or medical treatments). Whitton et al. (2016) reported that there are several ways to improve the appearance of vitiligo but the effect is limited. Narrowband ultraviolet B (NB-UVB) phototherapy is problematic, because the resulting repigmentation is transient. Skin grafting techniques are known to be the most effective interventions, but they can only be used with stabilized or segmental types of vitiligo, which are less common. In summary, there is currently no satisfactory solution to vitiligo and the patients are impacted for life. Therefore, the study of local knowledge of natural resources has become increasingly important in the investigation of the development of medicines without side effects (Min et al., 2017; Manurung et al., 2019; Chen et al., 2018). Many studies are underway to find potential pigment for the treatment of vitiligo and, Piper nigrum L. fruit extract has been shown to have growth-stimulatory activity in melanocytes (Lin et al., 1999). In addition, the natural resource extracts were studied Cucumis melo extract (Schallreuter et al., 2011) and Ammi visnaga fruits (Sidi and Bourgeois-Gavardin, 1952). Wood extracts were also tested as a treatment for vitiligo. In the pilot study of Szczurko et al. (2011) and Abu-Raghif et al. (2013), the potency of oral Ginkgo biloba extract to halt progression of active vitiligo was evaluated. Lin et al. (1999) found that out of 28 herbal extracts screened, significant stimulation (p<0.05) of melanocyte proliferation was observed using aqueous extracts of herbs. Tahir et al. (2010) reported that polylpodium leucotomos extract has been used for the treatment of vitiligo for more than 10 years in Europe. Madhogaria and Ahmed (2010) reported a patient who developed depigmented patches after using a cream containing kojic dipalmitate, licorice root extract, and Mitracarpus scaber extract. Rhododendron schlippenbachii (R. schlippenbachii) is widely distributed in Kurram Agency, Pakistan and the adjoining area in Afghanistan from 2000~3000 m. Some species of the genus R. schlippenbachii have been used as medicinal plants. The dried of R. schlippenbachii are used medicinally as an expectorant and treatment of acute-chromic bronchitis (Zhou et al., 1997).
From then R. schlippenbachii, systematic and comprehensive investigations of the genus Rhododendron were performed worldwide, from which hundreds of secondary metabolites have been isolated, mainly flavonoids and diterpenoids. Some of the isolates show various kinds of significant bioactivities (Chen et al., 2008). In addition, quercetin, a component of R. schlippenbachii extract, was reported to induce the upregulation of melanogenesis and enhance tyrosinase activity in dose- and timedependent manners (Nagata et al., 2004). Therefore, R. schlippenbachii extract could be used as a potential resource for plant-based pharmaceutical products for melanogenesis. In vitro and in vivo experiments were conducted to determine the effect of R. schlippenbachii extract on vitiligo treatment. The cytotoxicity, tyrosinase activity, and melanin content were analyzed in B16 melanoma cells in vitro. The melanin content, eumelanin content, and histologic analysis were examined in C57BL/6J Ler-vit/vit. A mice model for vitiligo, an acquired cutaneous depigmentary disorder, has been established and given the provisional genetic designation C57BL/6J Ler-vit/vit on Boissy et al. (1987). Through this study, we aimed to confirm the potential of R. schlippenbachii for the treatment of vitiligo.
2. MATERIALS and METHODS
The plant materials were obtained from the experiment forest of Gyeongsang National University, Jinju, South Korea. The fresh stem of R. schlippenbachii was cut into small pieces (about length 3 cm) and dried overnight at room temperature. The dried, R. schlippenbachii stem (500 g) were soaked in 98% ethanol 10 L for 1 week at room temperature. After vacuum filtration (Whatman No. 2 filter paper, ADVANTEC), the residue was extracted twice more in the same way as above. The filtrates were evaporated at 45°C under reduced pressure using a rotary evaporator to remove the solvent and lyophilized to obtain the crude extract at a yield of 21.8% (109 g). The extract was stored at 4°C before further processing.
B16 melanomas cell lines were provided by the dermatology of Ajou University Medical Center (164, World Cup-ro, Yeongtong-gu, Suwon, South Korea). The B16 melanomas growth medium was composed of Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA), 10% fetal bovin serum (FBS) and 1% penicillin/streptomycin (PS). The culture condition was 37°C in a humidif ied atmosphere with 0.5% CO2.
Subcultures of B16 cells were seeded in 96 well plates at a density of 7×103 B16 cells and cultured for 24 hours. The medium was then replaced with 500 μL fresh DMEM medium containing 10% FBS and 1% PS. 1μL(5, 10, 20μg R. schlippenbachii ethanol crude extract /1 mL 60% ethanol) of the R. schlippenbachii ethanol extract were added to each well and cultured for 3 days. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was dissolved in phosphate-bufferedsaline (PBS). After 3 days culturing, aliquots of MTT (at a final concentration of 5 mg/mL) were added 50 μL and then the cells were incubated in a 0.5% CO2 incubator at 37°C for 1 hour. The plates were then shaken with dimethylsulfoxide (DMSO) for 15 min to dissolve the blue/purple formazan crystals. The percentage of viable cells was quantified by identifying the ability to reduce MTT. The optical density was measured in an ELISA reader (Tetertek Multiskan MCC/340, Labsystem, Helsinki, Finland) at 540nm (Ahn et al., 2018).
The assay followed Kubo’s method (Kubo et al., 2004) with slight modification. Briefly, subcultures of B16 cells were seeded in 60 ø plates at a density of 8×104 B16 melanoma cell and cultured for 24 hours. The medium was then replaced with 3 mL fresh DMEM medium containing 10% FBS and 1% PS. The 1μL of the R. schlippenbachii ethanol extract were added to each well and cultured for 3 days. After 3 days culturing, the cells were harvested and suspended in 0.1 mL 1N NaOH-10% DMSO solution (v/v), kept at 60°C for 6 hours in water bath. The 90 μL test solution was transferred into 96 well plate and measured in an ELISA reader at 490nm. The melanin content was determined by calculation from a synthetic melanin standard curve.
Measurement of tyrosinase in L-DOPA oxidation of mushroom extracts was carried out quoted Masamoto et al. (2003). The experiment was performed by partially modifying the method. A 100 μL of 0.1 M phosphate buffer was mixed with 20 μL of different concentrations from R. schlippenbachii ethanol extract. Then, 20 μL of mushroom tyrosinase (2,000 U/mL in phosphate buffer) were added to initiate the reaction. The mixture was incubated at 37°C for 5 days and then incubated at 37°C for 10 min with the addition of 40 μL of L-DOPA (4 mM in 0.1 M phosphate buffer). The mixture was measured in an ELISA reader at 475 nm. The percentage activity of tyrosinase was calculated as follows: % activity = 100 - (B/A x 100), where A = ΔOD475 in 10 min without sample, and B=ΔOD475 in 10 min with tested sample.
Subcultures of B16 cells were seeded in 60 ø plates at a density of 8×104 B16 melanoma cell and cultured for 24 hours. The medium was then replaced with 3 mL fresh DMEM medium containing 10% FBS and 1% PS. The 1μL of the R. schlippenbachii ethanol extract were added to each well and cultured for 3 days. After 3 days culturing, the cells were harvested and lysed by incubation at –4°C for 1 hour in 100 μL lysis buffer (PBS pH 6.8, 1% trytone×100). The lysates were centrifuged at 10,000× g for 30 min (4°C) to obtain the supernatant as a source of tyrosinase. Tyrosinase activity was assayed as described previously (Masamoto et al., 2003). The reaction mixture contained 20 μg lysate, 180 μL 2 mM L-DOPA/pH 6.8 PBS and pH 6.8 PBS. After incubation in the presence at 37°C for 1 hour, Absorbance was measured at a wavelength of 490 nm to observe the dopachrome formation.
All the experimental procedures were performed according to the guidelines of the Committee for Ethical Usage of Experimental Animals at Gyeongsang National University. The C57BL/6J Ler-vit/vit mice with melanocyte disappearance (Medrano and Nordlund, 1990; Slominski and Paus, 1993) were used as in vivo animal models. Female C57BL/6J Ler-vit/vit mice were purchased from SAMTAKO (Gyeonggi-do, South Korea). These mice were stored under the conditions of temperature (20-2 6°C), humidity (30-70%) and illumination (lit from 08:00 to 20:00) and used for the experiment. The type of food for mice was standard diets and crude nutrients were 20% protein, 4.5% fat, 6% fiber, 7% ash, 0.5% calcium and 1% phosphorus. The bedding material was GLP bedding (SAMTAKO, Gyeonggi-do, South Korea) and number of cage companions was one. The C57BL/6J Ler-vit/vit mice used this experiment were 10-15 weeks of age. Food and tap water were provided ad libitum. R. schlippenbachii ethanol extract was dissolved in 60% ethanol and used in the experiment. The mice were randomly divided into two groups of five mice as group with mice treated 60% ethanol and mice treated 0.2 mL/cm2R. schlippenbachii ethanol extract for 5 months without intermission. The control was treated 60% ethanol on the opposite side of the same mouse. Repeated experiments were conducted with three sets and changes in hair color was monitored on once a day. When experiment finish, euthanization was performed by 10% isoflurane with prolonged exposure at 1, 2, and 4 h after administration, and death was confirmed by exsanguination.
Samples of hair were incubated overnight in 1M NaOH as previously described (Green and Wilson, 1996). Standards were prepared by dissolving synthetic melanin (Sigma Chemical Co., Poole, Dorset, U.K.) over the concentration range 0.05~0.4 mg/mL in 1M NaOH. The absorbance at 500 nm (total melanin) and 650 nm (eumelanin) of both standards and sample digests was measured using a Pye Unicam SP8-100 ultraviolet-visible spectrophotometer. It should be noted that synthetic and endogenous melanin differ in structure, and hence, values presented are comparative rather than absolute.
Throughout this investigation, the standard procedure of Laidlaw and Blackberg (1932) was used, and for carrying out the reaction both freshly prepared sheets of pure epidermis and frozen sections were employed. The specimens comprising the full thickness of the skin were subjected to Dopa treatment after a brief preliminary period of formol fixation. They were then given an additional period in the fixative and sectioned by ordinary methods. Incubation the split with EDTA solution (pH 7.4) was carried out at 37°C for 2 hours. Dermo-epidermal was separated with microforcep and washing with saline for 1 min. And than, the dermoepidermal was incubated with the L-dopa solution at 37 °C for 1 hour. After change the final L-dopa solution, dermo-epidermal was once more incubation at 37 °C for 8 hours and washing with saline for 1 min. Dyed dermo-epidermal was fixation with 10% formalin for 20 min and washing with distilled water for 3 min. Finally, dermo-epidermal was dehydration with 95% alcohol and 100% alcohol one by one for 20 min and clearing with the xylene for 20 min (three times). Dermo-epidermal on slide glass, it was mounting with canada balsam.
Chromatographic analysis was carried out by DAD following RP-HPLC separation installed with HIQ SIL C18V reversed-phase column (ø 4.6 mm × 250 mm) packed with 5μm diameter particles, the mobile phase was methanol-acetonitrile-water (40:15:45, v/v/v) containing 1.0 % acetic acid. Flow rate and injection volume were 1.0 mL/min and 10 μL, respectively. The sample were filtered through a 0.45 μm membrane filter. HPLC analysis was performed at ambient temperature, and the peak analysis of the chromatography was confirmed by comparison with the retention time of the cordycepin standard.
All the experiments were run at least in triplicate. SPSS 11.5 (SPSS Inc. Chicago) and PROC GLM in SAS 9.1 software (SASs Inc., Cary, NC) were used for all the statistical analysis: a descriptive statistical analysis was made by calculating the mean and standard deviation and comparison between groups was complemented by a comparison between means (pairwise t-test). P <0.05 and p <0.001 are interpreted as significant.
3. Results and Discussion
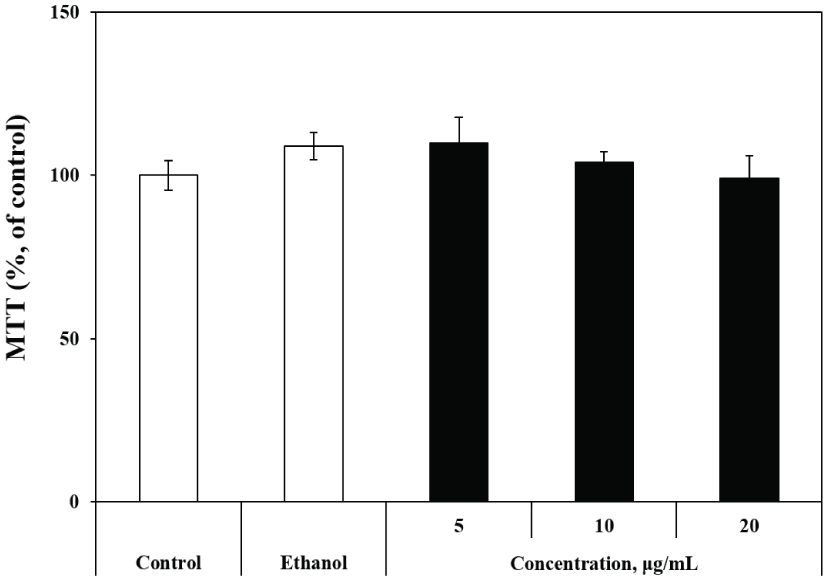
To evaluate the effects of the extract on cell proliferation, we investigated the effects of the extract on cell growth. The cells were exposed to various doses of extract for 72 h and cytotoxicity was determined by the MTT assay. As shown in Fig. 1, cell viability was maintained for 72 h after exposure to R. schlippenbachii extract. The cells were treated with various concentrations of R. schlippenbachii extract (5, 10, and 20 μg/mL) and the cell viability was calculated relative to the control. Cell viability was maintained as R. schlippenbachii extract concentration increased. These results indicated that R. schlippenbachii extract effectively induced the survival of B16 cells. In addition, R. schlippenbachii extract did not exert cytotoxic effects on B16 melanoma cell proliferation. The cell viability of control, treated with 60% ethanol and treated various concentration of R. schlippenbachii extract were not significantly different. Mosmann (1983) reported that the amount of formazan produced in the MTT assay is exactly proportional to the viability of the cells. Therefore, we subsequently examined the effects of R. schlippenbachii extract on melanin synthesis and cellular tyrosinase activity.
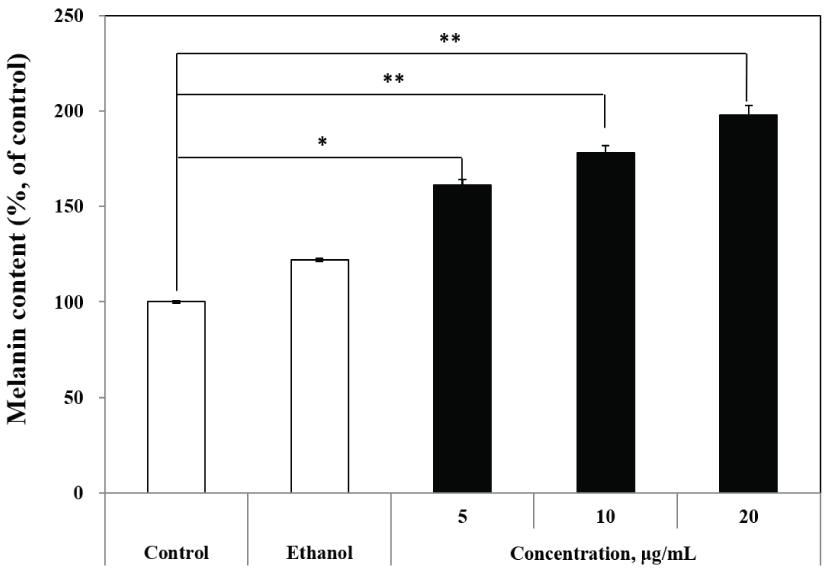
B16 melanoma cells offer quantifiable markers of cytodifferentiation, such as melanin production, as well as a morphological marker (dendrite formation) (Pomerantz, 1964). There is considerable experimental evidence to indicate that the processes of growth and melanization are intimately related in melanoma cells (Huberman and Callaham, 1979; Siracký et al., 1984). Lan et al. (2005) reported that melanin content was significantly lower in vitiligo lesions. Itoh and Furuichi (2005) used melanin content as an indicator for the evaluation of anti-graying effects in hair and improvements in vitiligo vulgaris. Niu et al. (2016) reported that the main cause of vitiligo was anti-melanogenic activity and that the analysis of melanin content was essential to improve vitiligo. To examine the melanogenic activity of the R. schlippenbachii extract, the stimulatory effect of R. schlippenbachii extract on melanin was evaluated in B16 melanoma cells. The B16 melanoma cells were treated with the R. schlippenbachii extract at 5, 10, and 20 μg/mL for 72 h. The melanin content was presented as a percentage of the control (vehicle). The following effects of R. schlippenbachii extract on melanogenesis of the B16 melanoma cells was found: R. schlippenbachii ethanol extract exerted a marked stimulatory effect on melanogenesis, without affecting cell proliferation, at concentrations of 5–20 μg/mL (Fig. 2). The R. schlippenbachii extract exhibited a significant dose-dependent increase on melanin content. The melanin content was 142.11% ± 0.07%, 180.00% ± 0.10%, and 181.95% ± 0.38% after treatment with 5, 10, and 20 μg/mL R. schlippenbachii extract, respectively. Kang et al. (2018) reported that the melanin content was 146% and 110% after treatment with 8 μg/mL and 40 μg/mL of Euphorbia supina extract in B16F10 cells, respectively. After the addition of 0.5 mM glycyrrhizin, the cellular melanin content reaches approximately 160% of control cells (Jung et al., 2001). The Tunisian C. spinosa extract has the ability to stimulate melanogenesis in B16 cells and has been found to increase melanin content by 12% and 60% at 0.005% and 0.05% extract concentrations, respectively (Matsuyama et al., 2009). The R. schlippenbachii extracts were confirmed to result in similar levels of melanin as previous studies, with a maximum melanin content of 181.95% after treatment with 20 μg/mL extract. Therefore, the R. schlippenbachii extract has the potential as a new natural resource to increase melanin content. Hamid et al. (2012) reported that the dark-black color of the B16F1 melanoma cell pellets demonstrated that α-MSH, forskolin, and mangosteen leaf extract stimulated melanogenesis activity. Nair et al. (2001) evaluated repigmentation, as determined by cell pellet color and melanin assays. In previous paper, the colors of cell pellets were evaluated visually for melanin content assay (Usuki et al., 2003). Therefore, these observations suggest that the R. schlippenbachii extract increased the melanogenic activity of B16 melanoma cells.
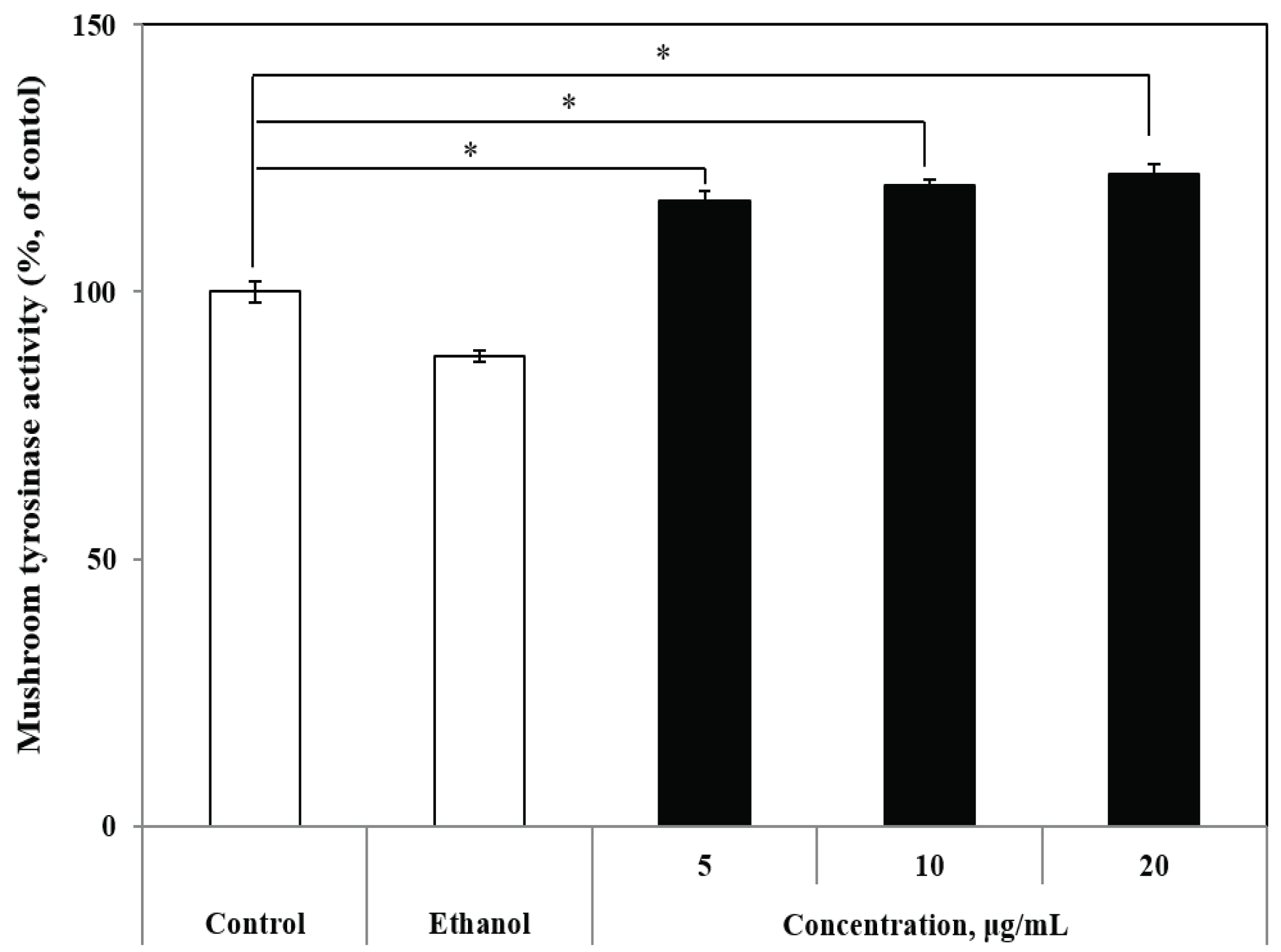
To determine whether R. schlippenbachii ethanol extract had a direct effect on the major enzyme in the melanogenesis, an in vitro cell-free mushroom tyrosinase assay was conducted. The effects of R. schlippenbachii ethanol extract on mushroom tyrosinase activity are shown in Fig. 3. We observed that the effects of the extract on the oxidation of L-DOPA by mushroom tyrosinase occurred in a dose-dependent manner. At 10 μg/mL and 20 μg/mL, R. schlippenbachii ethanol extract exerted activity on the oxidation of L-DOPA by mushroom tyrosinase; the 10 μg/mL R. schlippenbachii ethanol extract showed lower activity than the 20 μg/mL R. schlippenbachii ethanol extract. However, there was no significant difference between the two concentrations. Tyrosinase catalyzes 3,4- dihydroxyphenylalanine (DOPA) quinone formation from DOPA, and melanin formation from DOPA quinone via autoxidation and enzymatic reaction (Jimenez-Cervantes et al., 1993). Therefore, melanin production is related to tyrosinase expression; our results also showed the similarity. More specifically, both the melanin content and tyrosinase activity were increased by 10 μg/mL and 20 μg/mL R. schlippenbachii extract (Fig. 2 and Fig. 3). Most studies have been performed on the effect of the mushroom tyrosinase from various plant extracts (Yoshimura et al., 2005; Kim et al., 2003). Thus, R. schlippenbachii extract may be noted as an effective material with mushroom tyrosinase activity.
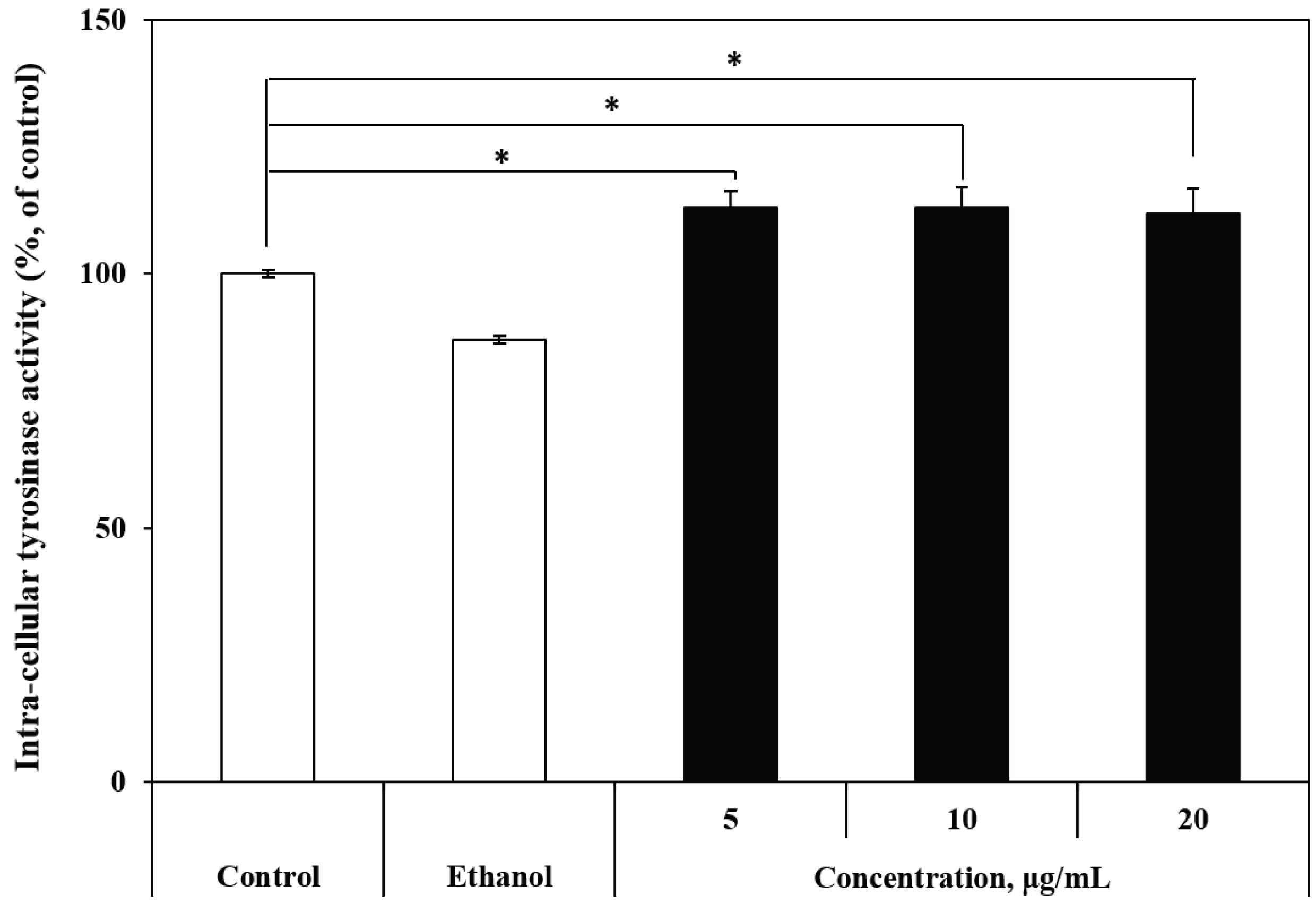
Tyrosinase catalyzes catalyze three steps in the biosynthesis process of melanin; hence, the measurement of tyrosinase activity is very important. The intracellular tyrosinase activity was measured after the culture of B16 melanoma cells with R. schlippenbachii ethanol extract. Different concentrations of R. schlippenbachii extract, not mushroom tyrosinase, were used to treat cell lysats extracted from B 16 melanoma cells. Equal masses of cell lysate were prepared with respect to the protein concentration. At 5 μg/mL R. schlippenbachii extract, a weak effect on the direct activation of intracellular tyrosinase was observed, but tyrosinaseinducing activity was increased significantly by 10 μg/mL and 20 μg/mL R. schlippenbachii extract (Fig. 4). Chen et al. (2012) showed that tyrosinase was regarded as the rate-limiting enzyme of melanogenesis, which modulates this process through the catalysis of the hydroxylation of tyrosine into DOPA and the further oxidation of DOPA into dopaquinone. Matsuda et al. (2005) also confirmed the potential stimulation of melanogenesis from tyrosinase activity by using cultured B16 melanoma cells. The activity of tyrosinase in melanocytes may be expressed in tyrosinase cells due to direct activity or an increase in the total amount of protein in the cell (Oh et al., 2011). In the previous results, we found that R. schlippenbachii extract exerted a significant influence on tyrosinase activity. This result shows that the R. schlippenbachii extract can be used directly as a tyrosinase activator. In a previous study (Jung et al., 2001), the cellular tyrosinase activity was also increased dose-dependently by glycyrrhizin, reaching 220% of the value in control cells at a treatment concentration of 1 mM. Adzuki bean extract is known to have a weak effect on the direct activation of tyrosinase in cells (Itoh and Furuichi, 2005). Tuerxuntayi et al. (2014) reported that, compared with untreated conditions, treatment with Kaliziri extract at 5–40 μg/mL resulted in a dose-dependent increase in tyrosinase activity in B16 cells (to a maximum of 138% tyrosinase activity). Treatment with forskolin significantly increased the intracellular tyrosinase activity by more than threefold, whereas treatment with 32 μg/mL extract clearly demonstrated a four-fold increase in intracellular tyrosinase activity (Hamid et al., 2012). These results suggested that R. schlippenbachii extract can be used as a stimulant of tyrosinase, similar to other natural extracts, in previous studies; moreover, they found that melanin synthesis activated as tyrosinase activity increased.
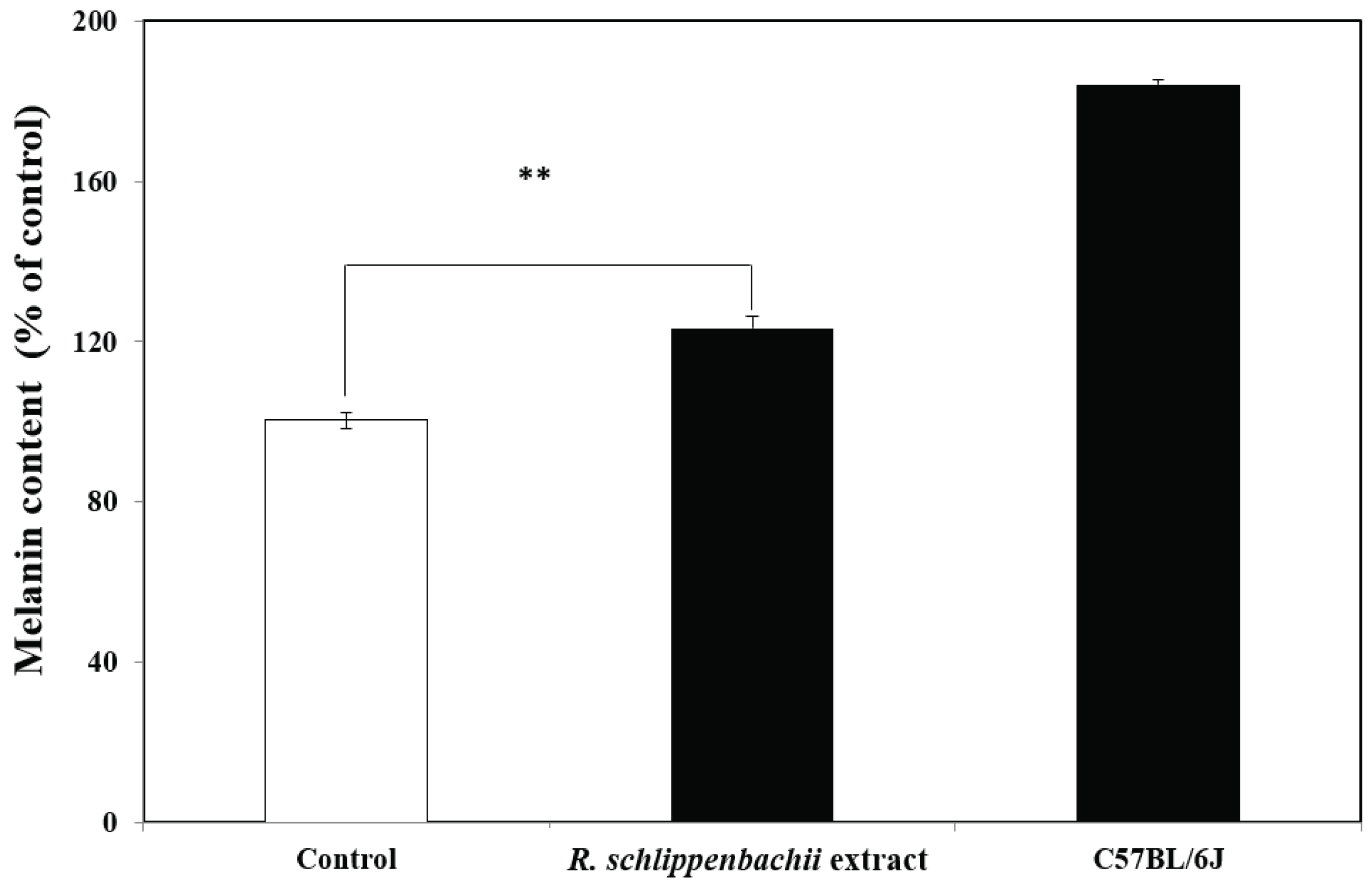
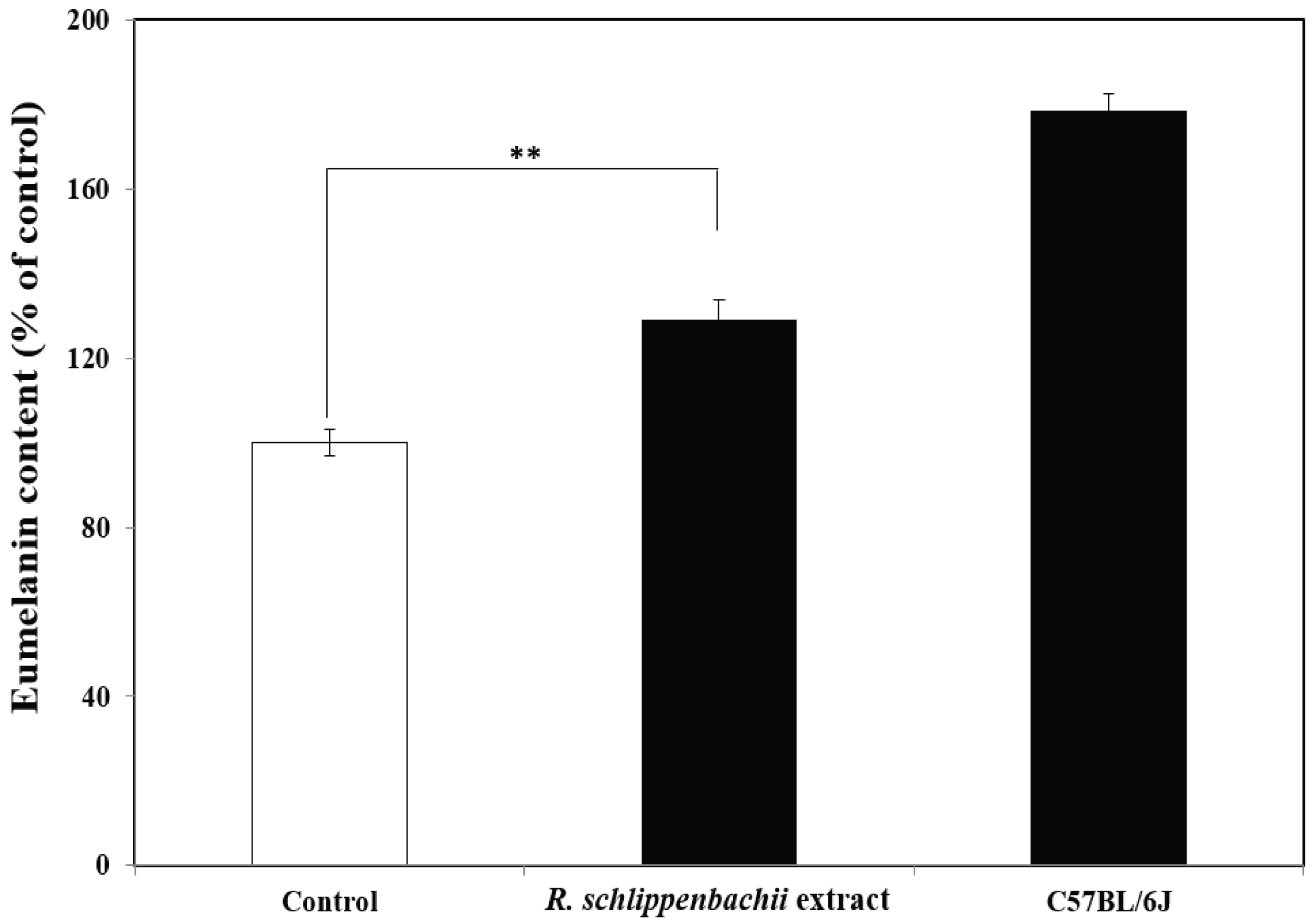
The experiment was conducted using a total of 15 mice, which were used for all experiments. Melanin contents in the hair samples taken from the back of C57BL/6J Ler-vit/vit mice were significantly higher in those treated with R. schlippenbachii ethanol extract than in the control group (p < 0.01) (Fig. 5). Therefore, the R. schlippenbachii extract has the potential to assist repigmentation in vitiligo, because it increased melanin content by approximately 40% compared with the control. In vertebrates and higher mammals, melanin plays an important role in thermoregulation, gastrointestinal tract, sexual attraction, and photoprotection. These melanin pigments can be distinguished chemically by the redyellow pheomelanin and the brown-black eumelanin. Both types of melanin are found in human hair, the epidermis, and cultured melanocytes. The main synthesis process for these two pigments is similar and is controlled by tyrosinase (Nogueira et al., 2007). However, the production of eumelanin is important for repigmentation in the treatment of vitiligo. The effect of R. schlippenbachii extract on the eumelanin content of mouse hair is shown in Fig. 6: treatment with R. schlippenbachii extract resulted in significantly higher eumelanin content than the control (60% ethanol) in the hair of C57BL/6J Ler-vit/vit mice hair (p < 0.01). Although treatment with R. schlippenbachii extract resulted in a lower eumelanin content than the hair of the normal C57BL/6J mice, we confirmed that the R. schlippenbachii extract was effective in increasing the eumelanin content in mice hair. Yonemoto et al. (1983) reported a decrease in eumelanin content in the lesions of vitiligo induced by 4-tertiary butyl catechol. Vitiligo occurs when there is a decrease in melanocytes and, in particular, when eumelanin is not formed (Prasad et al., 2003). Vitiligo can affect not only the skin but also the head and other parts of the body (Mihăilă et al., 2019). Thus, we suggest that R. schlippenbachii extracts can be effectively used for repigmentation in the treatment of vitiligo.
Melanin cells around hair follicles observed under an optical microscope correspond, by their localization, to the DOPA-positive cells observed under the optical microscope. Their morphological features are characteristic of melanocytes. In Fig. 7, representative sections of C57BL/6J Ler-vit/vit mice skin stained for melanin are shown. Microscopic examination revealed pigmented areas, with more repigmentation after treatment with R. schlippenbachii ethanol extract than the 60% ethanol used as a control. As shown by the staining, R. schlippenbachii extract promoted a melanocytic response and the concentration required for the promotion of pigmentation was 0.2 mL/cm2. These observations confirmed that R. schlippenbachii extract restored vitiligo pigmentation. Interestingly, R. schlippenbachii extracts cause a lot of pigmentation around mice hair follicles. The presence of melanocytes in the surrounding the root of mice hair follicles has already been documented (Ito and Wakamatsu, 2003). This was consistent with the scientific literature affirming that repigmentation tended to occur mainly in the areas of skin where there were still pigmented hairs, as this suggests the presence of melanin reservoirs (Menon et al., 2016). Thus, the repigmentation of the epidermis following dermabrasion originates from the melanotic portion of the hair follicle and it has been confirmed that R. schlippenbachii extract was an effective stimulator of the melanin stored around the hair follicles.
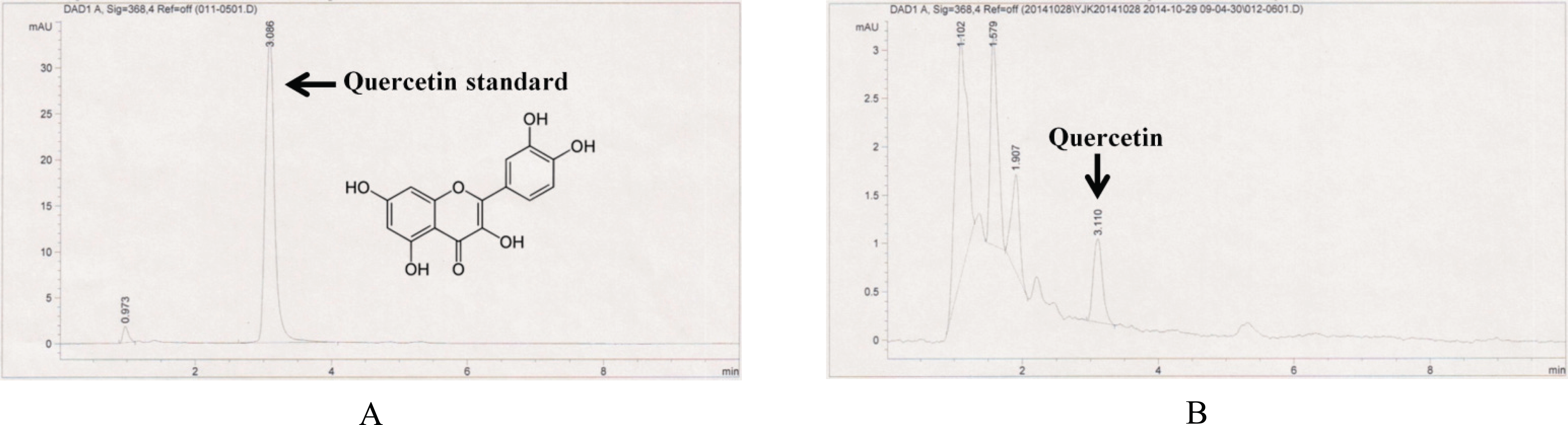
A quercetin was previously isolated as the principal regulator of tyrosinase from the dried flower of Heterotheca inuloides Cass (Compositae), known as “arnica” in Mexico (RodrÍguez-chÁvez et al., 2015; Kwon et al., 2009; Lee et al., 2004). In addition, quercetin was previously reported to control the oxidation of l-3,4-dihydroxyphenylalanine (L-DOPA, 2) catalyzed by mushroom tyrosinase (Kubo et al., 1994). Studies have investigated the effects of quercetin on tyrosinase activity and melanogenesis (Takekoshi et al., 2013). Nagata et al. (2004) demonstrated that the treatment of cultured melanoma cells with quercetin enhanced melanogenesis and also increased tyrosinase activity. We performed HPLC analysis to determine if quercetin was contained in the R. schlippenbachii extracts that we produced. The application of HPLC to the study of quercetin in R. schlippenbachii extract is shown in Fig. 8: the R. schlippenbachii extract had the same retention time as the quercetin standard, which meant that quercetin was present in the R. schlippenbachii extract. This was similar to previous studies that reported quercetin was contained in R. schlippenbachii extracts (Glyzin et al., 1970). Therefore, it appears that quercetin plays a role in the melanogenic activity of R. schlippenbachii extracts. Further, we have identified the potential of R. schlippenbachii extract to be an effective source of quercetin for melanogenesis.
In this study, the effects of extract from R. schlippenbachii on melanogenesis and tyrosinase activity were evaluated in B16 melanoma cells and C57BL/6J Ler-vit/vit mice. The results clearly demonstrated the R. schlippenbachii extract enhanced melanogenesis and also increased tyrosinase activity in cultured melanoma cells and C57BL/6J Ler-vit/vit mice. It is anticipated that continued research will increase knowledge concerning the activity of tyrosinase in R. schlippenbachii ethanol extract, and continue to shed light on therapeutic strategies that can be used to reduce or eliminate vitiligo. Unfortunately, there is little research about the activity of the mushroom tyrosinase in plant extracts. In addition, treatment with R. schlippenbachii extract led to a higher content of melanin and eumelanin in C57BL/6J Ler-vit/vit mice hair than in control (60% ethanol) mice, which demonstrated the therapeutic effect of hair-graying associated with vitiligo. Finally, we confirmed a notable increase in melanocytes in the skin of C57BL/6J Ler-vit/vit mice treated with R. schlippenbachii extract compared with the control. This study provides experimental evidence that R. schlippenbachii could be used as an effective treatment for the treatment of vitiligo and other skin diseases. However, further studies are necessary to establish specific details, such as how long the pigmentation lasts, what happens if the application is interrupted, how skin type and color influence the results, and effect for vitiligo treatment of other components excepted quercetin. In conclusion, R. schlippenbachii extract enhanced tyrosinase activities in mushroom tyrosinase and B16 melanoma cells. In addition, the extract was increased melanin content in melanocytes in both the B16 melanoma cells and C57BL/6J Ler-vit/vit mice hair. This study provides experimental evidence that R. schlippenbachii extract will be available as a therapeutic agent to relieve vitiligo.
