1. INTRODUCTION
Cellulose is a biopolymer made of glucose, and it is used as a structural biopolymer that is difficult to break down in ecosystems. Cellulase is a collective enzyme that converts cellulose to glucose with three kinds of cellulase: endo-β-1,4-glucanase (EG; EC 3.2.1.4), cellobiohydrolase (CBH; EC 3.2.1.74), and β-glucosidase (BGL; EC 3.2.1.21) (Goyal et al., 1991; Hong et al., 2001; Li et al., 2006; Kim et al., 2015a). For efficient cellulose degradation, EG, CBH, and BGL degrade cellulose enzymatically in sequence (Ali et al., 2013; Biswas et al., 2011; Gao et al., 2014; Haigler and Weimer, 1991; Howard et al., 2002; Schülein, 1988; Zhu et al., 2009). EG randomly hydrolyzes the amorphous regions of cellulose to produce oligosaccharides of various lengths. CBH hydrolyzes the end of cellulose oligosaccharide chains produced by EG and produces cellobiose units. Cellobioses produced by CBH are converted into two glucoses by BGL. The enzymatic efficiency of cellulose is enhanced by the activity and complementary action of enzymes of each type. Many studies showed that wood-rotting fungi degrade cellulose efficiently by the complementary action of cellulase. Therefore, many commercial cellulases are produced from wood-rotting fungi such as Trichoderma viride, Trichoderma koningii, Penicillium pinophilum, Sporotrichum pulverulentum, Fusarium solani, Aspergillus niger, Trichoderma reesei, and Penicillium funiculosum (Castellanos et al., 1995; Fang et al., 2010; Gao et al., 2008; Howard et al., 2002; Jamshidian et al., 2016; Leathers et al., 2015; Lee et al., 2010; Pandey et al., 2006; Smits et al., 1996; Sutivisedsak et al., 2013; Zhang et al., 2012).
Among fungi, T. reesei has already been studied and used extensively in the production of commercial enzymes (Goldbeck et al., 2013; Howard et al., 2002; Jørgensen et al., 2003; Kurabi et al., 2005). Trichoderma has been reported to be suitable for the production of industrial cellulase because its ability to produce extracellular enzymes is greater than that of bacteria (Rosgaard et al., 2006). T. reesei, however, cannot break down lignin (Howard et al., 2002) and the BGL of T. reesei is partially bound to the mycelium, so BGL is not efficiently recovered during enzyme production (Delabona et al., 2012). In the case of Schizophyllum, it was reported that the extracellular polysaccharide schizophyllan is produced during cellulase production (Esterbauer et al., 1991; Jamshidian et al., 2016; Leathers et al., 2015). Schizophyllan increased the viscosity of culture with cellulase production by forming a jelly-like structure. It is difficult to produce highly purified extracellular cellulase because of the high viscosity of enzyme solution by schizophyllan. In general, good microbial strains for economic enzyme production have a high enzymatic activity and a high specific activity without production of impurities in culture medium. In this study, we report Acanthophysium sp. KMF001, which produced cellulase with high activity and high specific activity.
Acanthophysium is a fungus in the Stereaceae family and Basidiomycota phylum and is known to be widespread in nature, but the ecological role of Acanthophysium is still unclear.
2. MATERIALS and METHODS
Acanthophysium sp. KMF001 (KCTC 18282P) was deposited at Korea Collection for Type Culture (Jeongeup-si, Korea). Acanthophysium bisporum (MUCL 32213), Acanthophysium cerussatum (MUCL 32645), and Acanthophysium lividocaeruleum (MUCL 33688) were purchased from the Belgian Co-ordinated Collections of Micro-organisms (Ottignies-Louvainla- Neuve, Belgium) as comparative strains for cellulase activity.
Acanthophysium sp. KMF001 was activated on potato dextrose agar (catalog no. 7349834; BD Biosciences Korea, Seoul, Korea) plates at 30°C for 5 to 7 days. A. bisporum, A. cerussatum, and A. lividocaeruleum were activated on malt extract agar (20 g/L malt extract, 20 g/L glucose, 1 g/L peptone, and 20 g/L agar) plates at 25°C for 5 to 7 days.
The activated Acanthophysium sp. KMF001 was inoculated in 100 mL of potato dextrose broth (catalog no. 8129687; BD Biosciences Korea) and incubated at 30°C for 5 days under the shaking condition at 150 rpm to make a pre-culture. For A. bisporum, A. cerussatum, and A. lividocaeruleum, the activated cells were inoculated in 50 mL of malt extract broth (catalog no. 218630; BD Biosciences Korea) and incubated at 25°C for 5 days under the shaking condition at 150 rpm to make a pre-culture.
In the main culture, all four strains were inoculated with 5% (v/v) pre-culture in 200 mL modified TYE medium with cellulose [7 g/L tryptone, 3 g/L yeast extract, 5 g/L K2HPO4, 5 g/L KH2PO4, 3 g/L MgSO4·7H2O, and 20 g/L cellulose (20–100 mm; catalog no.25384405; Daejung, Seoul, Korea) at pH 6.0] in a 1 L baffled flask and incubated for 3 weeks under the shaking condition at 150 rpm at 30°C for Acanthophysium sp. KMF001 and at 25°C for A. bisporum, A. cerussatum, and A. lividocaeruleum.
The crude supernatant from each culture was collected, cells were removed by filtration using Whatman filter paper No. 1 (catalog no. 1001-110; GE Healthcare Life Sciences, Seoul, Korea). The cell free supernatant of each was analyzed on the cellulase activity.
For the EG activity assay, 5 μL of crude enzyme solution was mixed with 45 μL of substrate solution made with 2% (w/v) carboxylmethylcellulose sodium salt (catalog no. MKBW6753V; Sigma-Aldrich Korea LLC, Yongin, Korea) in 0.1 M sodium citrate buffer at pH 4.5. The mixture was incubated at 50°C for 30 minutes. The reaction was stopped by adding 50 μL of copper solution and heating at 100°C for 10 minutes. The amount of produced reducing end by EG was measured using the Somogyi–Nelson method (Nelson, 1944). One unit of EG was defined as the amount of enzyme producing 1 μmol glucose for 30 minutes under the described reaction condition.
For the CBH activity assay, 100 μL of crude enzyme solution was mixed with 100 μL of 10 mM p-nitrophenyl- β-D-cellobioside (catalog no. 077M4023V; Sigma-Aldrich Korea LLC) and 800 μL of 0.1 M sodium citrate buffer at pH 4.5. The mixture was incubated at 50°C for 15 minutes. The reaction was stopped by adding 100 μL of 2 M Na2CO3. The amount of produced p-nitrophenol by CBH was measured at 405 nm using a Opsys MR microplate reader (Hynex Technologies, Chantilly, VA, USA). One unit of CBH was defined as the amount of enzyme producing 1 μmol p-nitrophenol for 15 minutes under the described reaction condition.
For the BGL activity assay, all measuring procedure was similar with the above CBH activity assay with switching the substrate to 10 mM p-nitrophenyl-β-D-glycopyranoside (catalog no. BCBP2339V; Sigma-Aldrich Korea LLC). Enzymatic activity was defined as the enzyme activity of the crude solution based on the volume and expressed in U/mL.
To calculate a specific activity of each cellulase, the protein concentration was measured using the Bradford’s methods with bovine serum albumin as a standard (Bradford, 1976). Enzyme with 400 μL and protein assay reagent with 100 μL (Bio-Rad Laboratories, Inc., Hercules, CA, USA) were mixed violently in tubes for 10 seconds. The absorbance was measured at 595 nm after 5-minute incubation in room temperature. The specific activity of enzymes was defined as the cellulase activity per milligram of total protein and expressed in U/mg.
Cells of Acanthophysium sp. KMF001 from the main culture were disrupted with TissueLyser LT (Qiagen Korea Ltd., Seoul, Korea), and the genomic DNA was purified using the DNeasy Plant Mini kit (Qiagen Korea Ltd.). The internal transcribed spacer region of 28S rRNA was amplified using polymerase chain reaction (PCR) with two primers: ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′). The PCR condition with 35 cycles was 95°C for 20 seconds for denaturation, 55°C for 4 seconds for annealing, and 72°C for 60 seconds for extension. The amplified DNA was sequenced by Biofact Co., Ltd. (Daejeon, Korea). The analyzed sequence was registered at GenBank at the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/) under MM680758.
The homologous sequences in the nucleotide database were identified using Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). They were aligned, and phylogenetic trees were analyzed using MEGA4 software (Tamura et al., 2007). The corresponding ribosomal DNA region of each strain was used for making phylogenetic trees with the distance methods based on the similarity.
3. RESULTS and DISCUSSION
Acanthophysium sp. KMF001 was selected among 54 wood-rotting fungi based on the cellulase activity (Kim et al., 2015b). To evaluate the cellulase activity of Acanthophysium sp. KMF001, we purchased three Acanthophysium strains, namely, A. bisporum, A. cerussatum, and A. lividocaeruleum, and the cellulase activities of all four strains, including Acanthophysium sp. KMF001, were analyzed (Fig. 1). Cellulase production was analyzed on the basis of culture medium volume (Fig. 1A, 1C, and 1E) and protein weight for the specific activity (Fig 1B, 1D, and 1F). As shown in Fig. 1, A. cerussatum and A. lividocaeruleum showed low enzymatic and specific activity of cellulase, and Acanthophysium sp. KMF001 and A. bisporum showed strong cellulase activity among the four tested strains.
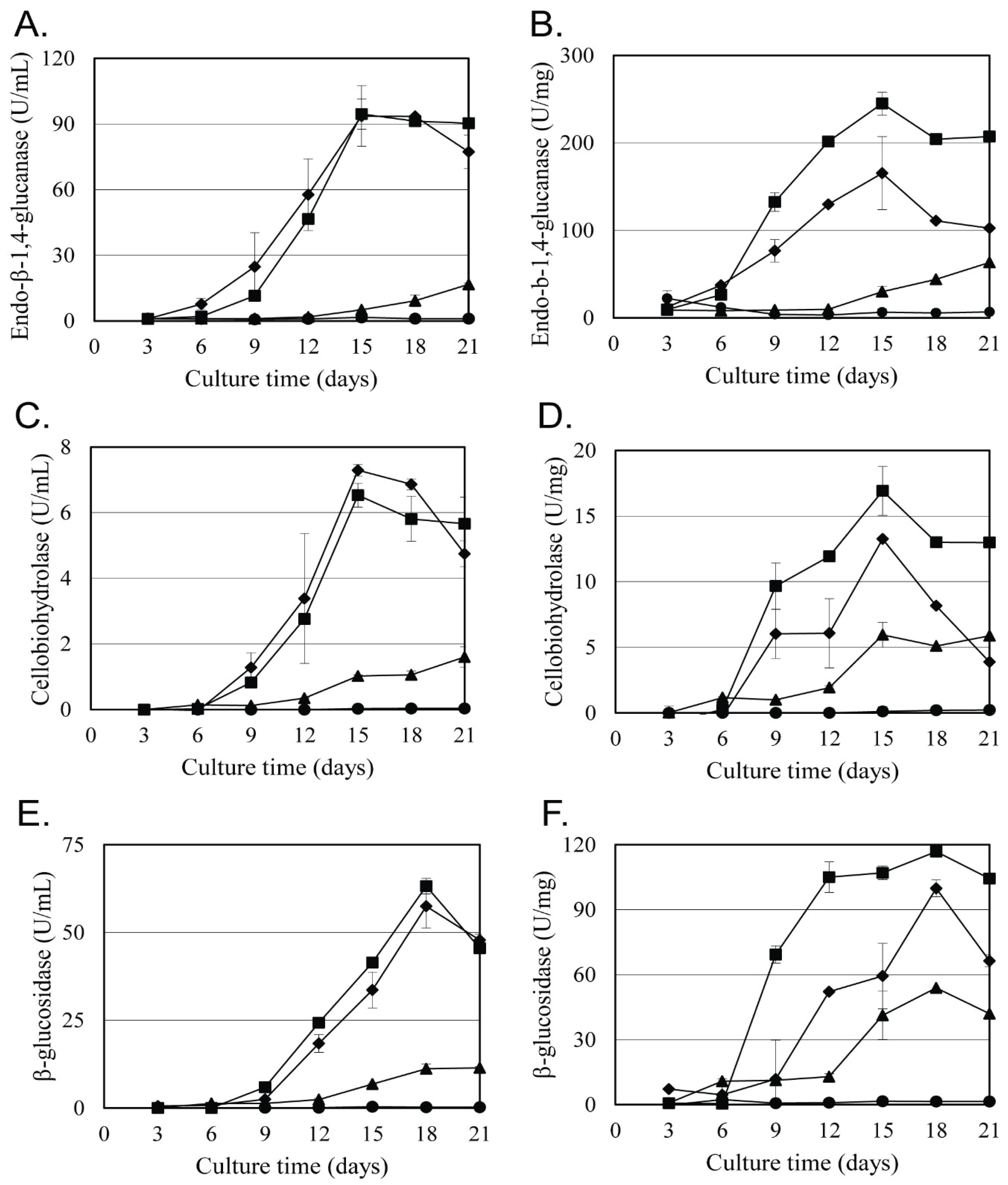
The EG activity of Acanthophysium sp. KMF001 showed the highest enzymatic activity at 94.54 U/mL on 15 days of cultivation and similar enzyme activity until 21 day. On the other hand, EG enzyme activity of A. bisporum showed a similar level of EG activity to Acanthophysium sp. KMF001 at 15 days of cultivation, but the enzyme activity decreased with further incubation time after that (Fig. 1A). However, the specific activities of EG in Acanthophysium sp. KMF001 and A. bisporum showed 245 U/mg and 165 U/mg, respectively, at the 15 days of cultivation (Fig. 1B). These results indicated that the enzyme productivity per unit volume of the culture was similar in both strains, but the cellulase content based in the protein released to the outside of the cell in Acanthophysium sp. KMF001 was 1.5 times higher than that in A. bisporum. The other two cellulase enzyme activities, namely, CBH (Fig. 1D) and BGL (Fig. 1F), showed similar differences in specific enzyme activities. The production of such high-purity cellulase is advantageous for enzyme production and application.
The CBH activities on the 15th day of cultivation with Acanthophysium sp. KMF001 and A. bisporum showed 6.83 U/mL and 7.19 U/mL, respectively (Fig. 1C). The BGL activities on the 18th day of cultivation in Acanthophysium sp. KMF001 and A. bisporum showed 63.20 U/mL and 57.46 U/mL, respectively (Fig. 1E). The specific activity of CBH and BGL in Acanthophysium sp. KMF001 was higher, with 17% and 28% more, respectively, than those in A. bisporum (Fig. 1D and 1F, respectively). In particular, on the 15th day of cultivation, the specific CBH activity in Acanthophysium sp. KMF001 was about double of that in A. bisporum (Fig. 1D), and the specific BGL activity in Acanthophysium sp. KMF001 on the 12th day of cultivation was also about double of that in A. bisporum (Fig. 1F). In the case of Acanthophysium sp. KMF001, the decrease in CBH activity was moderate after the 15th day of cultivation, but the CBH activity of A. bisporum rapidly decreased during the same incubation time.
On the basis of the above results, all enzymatic cellulase activities of Acanthophysium sp. KMF001 were similar to that of A. bisporum, but the specific activity of Acanthophysium sp. KMF001 showed significantly higher levels of all tested enzyme activities than A. bisporum. This observation suggested that Acanthophysium sp. KMF001 was better than the others in Acanthophysium spp. tested in this study for high cellulase production. In a previous study, the CBH and BGL activities of Acanthophysium sp. KMF001 were higher than it of Trichoderma reesei, Fomitopsis palustris, and Aspergillus niger (Kim et al., 2015b). Considering the enzyme activities in the culture solution and specific activities in this study, the 15th day of cultivation is ideal for cellulase production of Acanthophysium sp. KMF001. In a previous study, the crude cellulase of Acanthophysium sp. KMF001 improved the quality of fabric effectively (Shin et al., 2016). In addition to effective cellulase production, Acanthophysium sp. KMF001 produced a novel endo-β -1,4-xylanase, which was an enzyme for hemicellulose degradation (Yoon et al., 2018). All studies suggested that Acanthophysium sp. KMF001 was a good enzyme-producing fungus for decaying wood.
To identify Acanthophysium sp. KMF001 genetically, the genetic information of its 28S rRNA was analyzed (Fig. 2). The results of the BLAST search with the 28S rRNA nucleotide sequence of Acanthophysium sp. KMF001 using the NCBI nucleotide database are shown in Table 1. The most homologous strain was A. lividocaeruleum MB1825, with 98.40% identity. The next most homologous strains were Acanthophysellum lividocoeruleum, with 98.24% identity, and additional six strains with 97.92% identity (Table 1). Using phylogenetic analysis with strains in Table 1, the taxonomic position of Acanthophysium sp. KMF001 was analyzed in Fig. 3. The phylogenetic tree analysis revealed that Acanthophysium sp. KMF001 separated into different phylogenetic branches from A. lividocaeruleum MB1825, the most similar strain with 99% identity by Nucleotide BLAST search. Although Acanthophysium sp. KMF001 was similar to the previously reported A. lividocaeruleum MB1825 with an identity of 98.40%, the phylogenetic analysis suggested that Acanthophysium sp. KMF001 might be a new strain.

4. CONCLUSION
Comparison of the enzyme activity with other strains of Acanthophysium genus showed that Acanthophysium sp. KMF001 had similar levels of cellulase activity with A. bisporum in all three types, namely, EG, CBH, and BGL, but the specific enzyme activity of Acanthophysium sp. KMF001 was higher than cellulase from other tested Acanthophysium strains. Although Acanthophysium sp. KMF001 showed 98.40% nucleotide identity with the most homologous strain, the phylogenetic tree suggested that Acanthophysium sp. KMF001 is a new strain by separating into a different phylogenetic branch from the most homologous strain. This study suggested that Acanthophysium sp. KMF001 is a novel bacterium that produces an effective cellulase for the wood decay.
