1. INTRODUCTION
For the recent decades processes of biomass conversion into renewable fuels has been attracting much attention due to decreasing of crude-oil reserves, enhanced demand for fuels worldwide and increased climate concerns about the use of fossil-based energy carriers (Zhang et al., 2015; Kim 2016; Prajitno et al., 2016; Chiaramonti et al., 2017; Zhang et al., 2018). In contrast to fossil fuel reserves, biomass is indicated as an abundant, sustainable and carbon-neutral renewable energy resource for the production of biofuels and valuable chemicals.
In particular, Korea is the world's 10th largest emitter of carbon dioxide and should be reducing greenhouse gas emissions from 2013. According to the Korea Wood recycling association, the production of waste wood in Korea is about 5 million ton, and the waste from afforestation is the highest amount, 48% in 2005. The amount of waste wood decreased to 2 million ton in 2016, and 66% those waste wood is recycled, as charcoal or combustion in combined heat and power (CHP) system. The Korea ministry of environment established the law related to waste-to-energy and biomass-toenergy in 2009, and the Renewable Energy Portfolio Standard (RPS) was enforced from 2012.
Larch (Larix kaempferi) forests are the dominant forest type through northeastern Asia to central Siberia. The net ecosystem production (NEP) of a larch forest has been routinely measured by the eddy covariance method at the Tomakomai flux site since the autumn of 2000 (Hirano et al., 2003). In Hokkaido, Japan, larch plantations (470,000 ha) account for about one third of all forests, because of their high productivity (Liang et al., 2004).
Fast pyrolysis technology is commonly used for the biomass conversion into liquid products, referred to as bio-oil. The bio-oil composition depends on the type of feedstock (biomass) and the pyrolysis conditions (Bridgwater, 2012). However, the high content of oxygen usually leads to disadvantageous fuel features, like high viscosity, thermal and storage instability, corrosiveness, poor heating value and immiscibility (Wen et al., 2014; Long et al., 2015; Moon et al., 2016; Lee et al., 2016; Prajitno et al., 2016; Zhang et al., 2018) and makes it complicated for direct use (Lu et al., 2009). The key reaction of this process is the hydrogenolysis of C-O bonds (hydrodeoxygenation) of oxygen-containing compounds with the formation of water (Choudhary 2011; Mortensen et al., 2011).
Early work indicated that conventional hydrodesulphurization (HDS)/hydrodenitrogenation (HDN) catalysts exhibit promising activity in hydrodeoxygenation (HDO) of phenolic compounds such as phenol, anisole, and guaiacol (Bredenberg et al., 1982; Laurent et al., 1994b; Centeno et al., 1995; Ferrari et al., 2001; Buiet al., 2011a). However, these metal–sulfide catalysts suffer from deactivation in the presence of high water content and the continuous addition of sulfur is required in the reactant stream to maintain the catalysts in the sulfide form. This last factor in particular can cause serious problems for the downstream processes (Laurent 1994a; Ferrari et al., 2001; Zakzeski et al., 2010). Alternative hydrotreating catalysts have been sought for bio-oil upgrading (Elliott 2008; Wildschut et al., 2009; Zakzeski et al., 2010; González-Borja 2011; Nimmanwudipong et al., 2011b; Zhao et al., 2011; Zhu et al., 2011). Recently, Gates’ group compared Pt/Al2O3 and Pt/HY catalysts in the HDO of anisole and guaiacol. Their results showed that the transalkylation activity of the catalyst was significantly affected by the type of acidic site (Nimmanwudipong et al., 2011a; Nimmanwudipong et al., 2012). A later study by Zhu et al. further confirmed the effect of acidic sites on the transalkylation activity in the HDO of anisole (Zhu et al., 2011). A very recent report indicates that Fe/SiO2 shows a higher hydrodeoxygenation activity without saturation of aromatic ring in the guaiacol conversion (Olcese et al., 2012). While the transalkylationactivity of the acidic catalyst offers a way to preserve the carbon from being lost as a gas product, the presence of acidic sites interacts strongly with the phenolic compounds, resulting in rapid catalyst deactivation by coking (Centeno et al., 1995; Huber et al., 2006).
Moreover, bifunctional catalysts comprised by active metal and solid acid exhibits excellent activity for HDO reaction. Support material is also thekey factor determining the catalytic performance as well as active metal. ã-Al2O3, which was widely used for HDO due to cheap cost, excellent texture and suitable acidity, showed excellent HDO activity in the HDO of phenolic compounds (Bui et al., 2011b; Zhang et al., 2013), occurred high amount of coke formation as well as boehmite resulting decrease the catalytic activity. Therefore, finding the suitable catalysts to overcome those drawbacks is conducted in numerous studies. For example, Ni/HZSM-5, Pt/HZSM-5, Ru/HBeta and Pt/HBeta could effectively transformed guaiacol into hydrocarbons (Zhu et al., 2011; Zhao 2012; Ohta et al., 2015; Yao et al., 2015; Zhanget al., 2018).
Here, we describe the preparation and reactivity for a series of bifunctional catalysts for the HDO of waste wood bio-oil. Especially, effect of support zeolite with NiFe bimetallic catalysts on the heavy oil products after HDO process will be investigated by analyzing catalysts and product features.
2. MATERIALS and METHODS
Catalytically active transition metal sites (Ni and Fe) were added by means of a wet impregnation method (Yakovlev et al., 2009; Ardiyanti et al., 2012). Equal amounts of nitrate-form transition metal (Ni and Fe) solution and three zeolite supportswere stirred for 1 h. After the mixture was homogenized, it was dried overnight at 80 °C and calcined in air at 550 °C for 3 h to obtain NiFe/HBeta, NiFe/ZSM-5 and NiFe/Zeolite Y. The catalysts were reduced at 500 °C for 3 h under an H2 atmosphere before use. Two commercial catalysts (CoMo/Al2O3 and NiMo/Al2O3) were also performed HDO reaction to compare the activity.
The specific surface areas of the support and catalysts were measured by means of a Brunauer–Emmett–Teller (BET) method, and the pore distributions and cumulative pore volumes were calculated by means of the Barret–Joyner–Halenda (BJH) method, based upon the desorption branches of the N2 isotherms measured at 77 K. Samples were degassed at 523 K for 4 h before these tests.
Bio-oil obtained from fast pyrolysis of waste wood was offered from Yonsei University. A stainless steel reactor equipped with a thermocouple, a pressure sensor, and a system for controlling the temperature and pressure was used as the test chamber. 4 wt% of the reduced catalysts were added to a mixture of bio-oil and ethanol and then placed into the sealed reactor and flushed with N2. The reactor was then pressurized to 3 MPa H2, and the temperature was increased to the reaction temperature (300, 350, or 400 °C) (Laurent 1994b; Ferrari et al., 2001). The ethanol concentration varied 25, 10, and 5 wt% in the mixture. Three zeolite supportedcatalyst (NiFe/HBeta, NiFe/ ZSM-5 and NiFe/ Zeolite Y) and two commercial catalysts (CoMo/Al2O3 and NiMo/Al2O3) were performed HDO reaction to test the activity. After the reaction, three phases of four products were obtained: liquid (immiscible light oil and lower heavy oil), char, and gas. The upper solvent-based water-rich light oil and lower organic heavy oil were recovered after the reaction and separated using a separatory funnel. The yields of char, light oil and heavy oil were calculated according to the following equations.
To measure the water content and acidity (total acid number, TAN) of the bio-oil and heavy oils, Karl Fischer titration and ASTM D664 TAN testing were performed. Each sample was analyzed three times; values reported in tables herein represent the averages of the triplicate analyses. Viscosities were determined at 20 °C using a VIBRO viscometer (AND, Japan). The higher heating value (HHV) was calculated according to the following equation (Friedl et al., 2005):
where [C], [H], and [N] are the mass% of carbon, hydrogen, and nitrogen on dry basis, respectively. The results were corrected for the water content of the oil.
Atomic O/C and H/C were calculated to compare the hydrogenation and deoxygenation degree of each heavy oil; the degree of deoxygenation (DOD) was estimated as follows.
Here, MOheavy oil and MObio-oil are the molar oxygen/ carbon ratios of heavy oil and bio-oil.
For measuring low-molecular-weight compounds in bio-oil and heavy oils qualitatively and quantitatively, GC/MS analysis was performed with fluoranthene as an internal standard. The analysis was conducted with an Agilent 7890B coupled with a 5975C mass selective detector (MSD) and a flame ionization detector (FID) equipped with a DB-5 capillary column (60 m × 0.25 mm × 0.25 μm). The oven temperature was maintained at 50 °C for 5 min, followed by heating at a rate of 3 °C/min to 280 °C and holding for 20 min. The injector and FID detector temperatures were 250 and 300 °C, respectively.
3. RESULTS and DISCUSSION
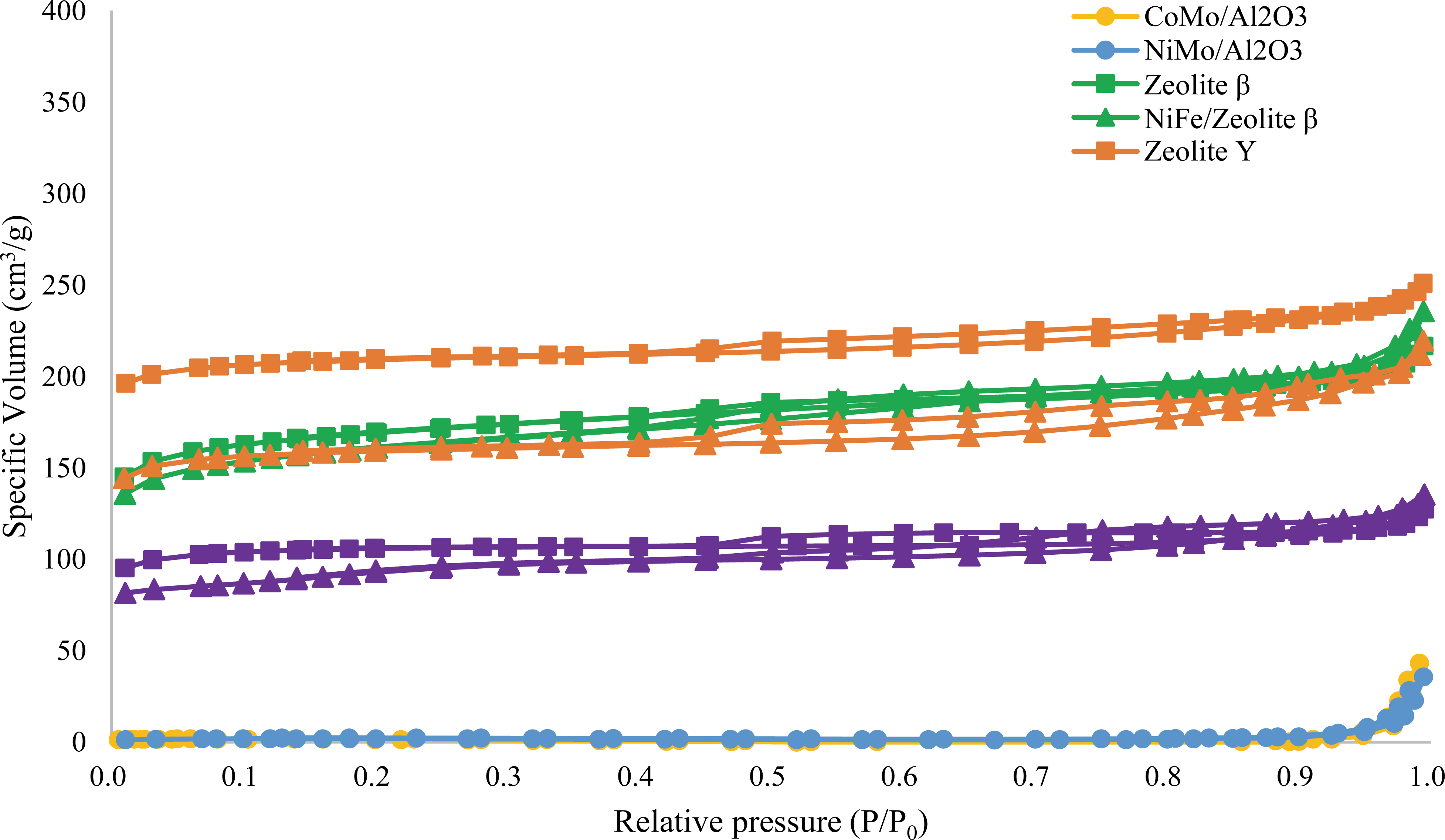
Table 1 presents the physical properties of the catalysts used in this study. The specific surface areas of NiFe/zeolite Y, NiFe/HBeta, and NiFe/ZSM-5 were 532.2, 545.1 and 316.5 m2/g, while the pores volumes were 0.3, 0.3 and 0.2 cm3/g, respectively. These values were lower than the supports themselves, which might be due to the metal loading (about 5 wt.%). As shown in Fig. 1, zeolite supports were mono-layer support with micro-pores (Lippens 1965). Isothermal type of the supports presented that the zeolite supports have tubular with short necks, wide sloping bodies or various widths and the shape of pores remained after the metal impregnation. However, CoMo/Al2O3 and NiMo/Al2O3 were not clearly defined their pore type, since they presented type III physisorption isotherm, which meant weak adsorption interaction, and didn’t match the hysteresis loops. Therefore, Zeolite supported bifunctional catalysts (NiFe/zeolite Y, NiFe/HBeta, and NiFe/ ZSM-5), with a high specific surface area and large pore, was expected to be effective for good dispersionof the reactant during the reaction. Compared with the zeolites and metal doped bifunctional catalysts, the pore diameter showed increase. It might be due to the crack of the pore during impregnation step (Munnik et al., 2015).
The morphologies of the catalysts were investigated by SEM (Fig. 2). Commercial CoMo/Al2O3 and NiMo/Al2O3 showed smaller particles than prepared NiFe-based catalysts. Moreover, according to Fig. 2 (c)-(e), zeolite supports presented different particle shapes, despite their pore type (shape) and size were similar (Fig. 1 and Table 1). NiFe/ZSM-5, which has lower specific surface area (316.5 m2/g) than other zeolite supported bifunctional catalysts (ranged between 532.2 to 545.1 m2/g) has rectangular large particles.
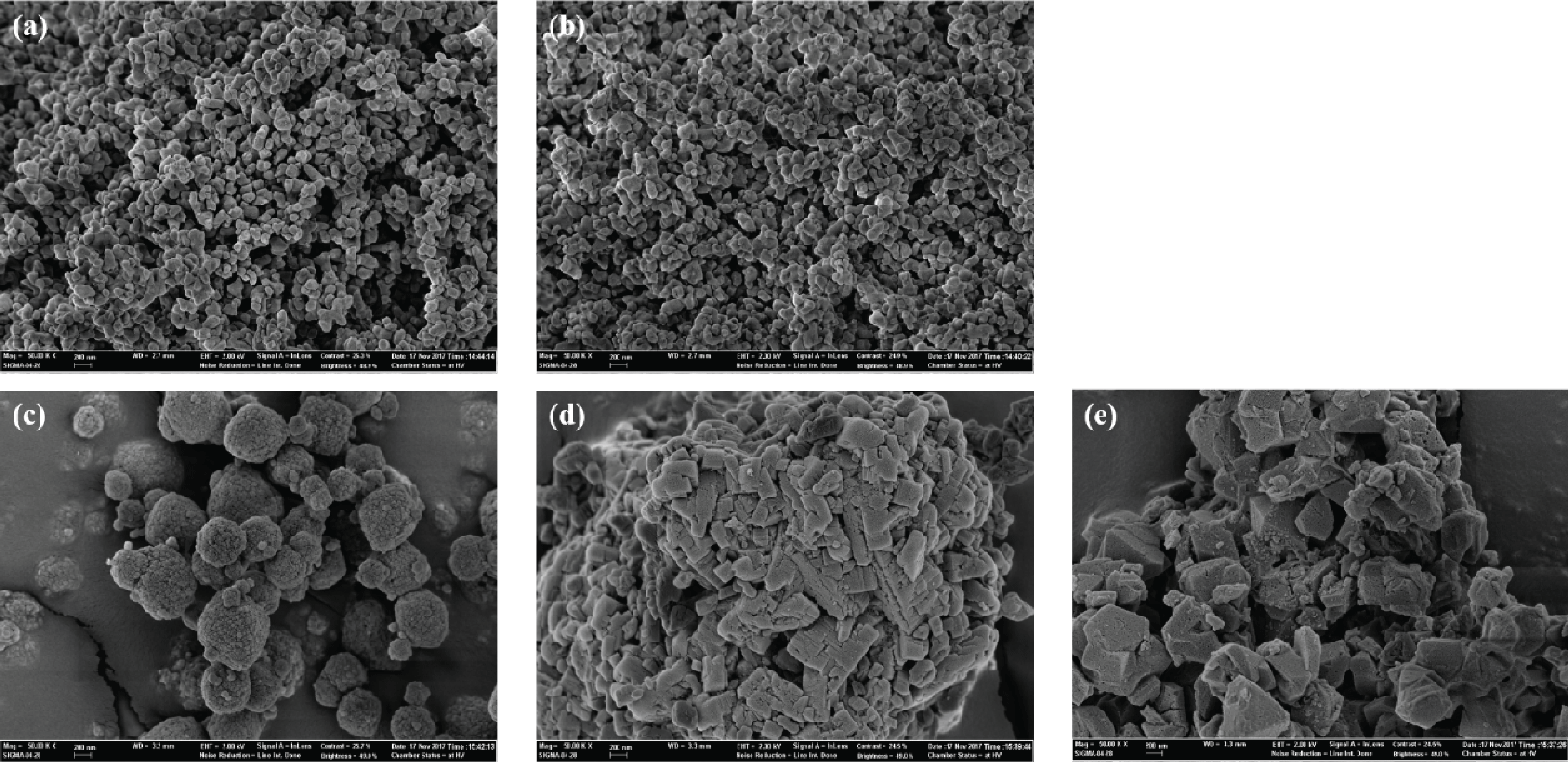
While in Fig. 3, the catalysts were lost its original shape after the reaction. Some reactant and produced coke were deposited on the catalyst surface. It might deactivate the catalyst activity.
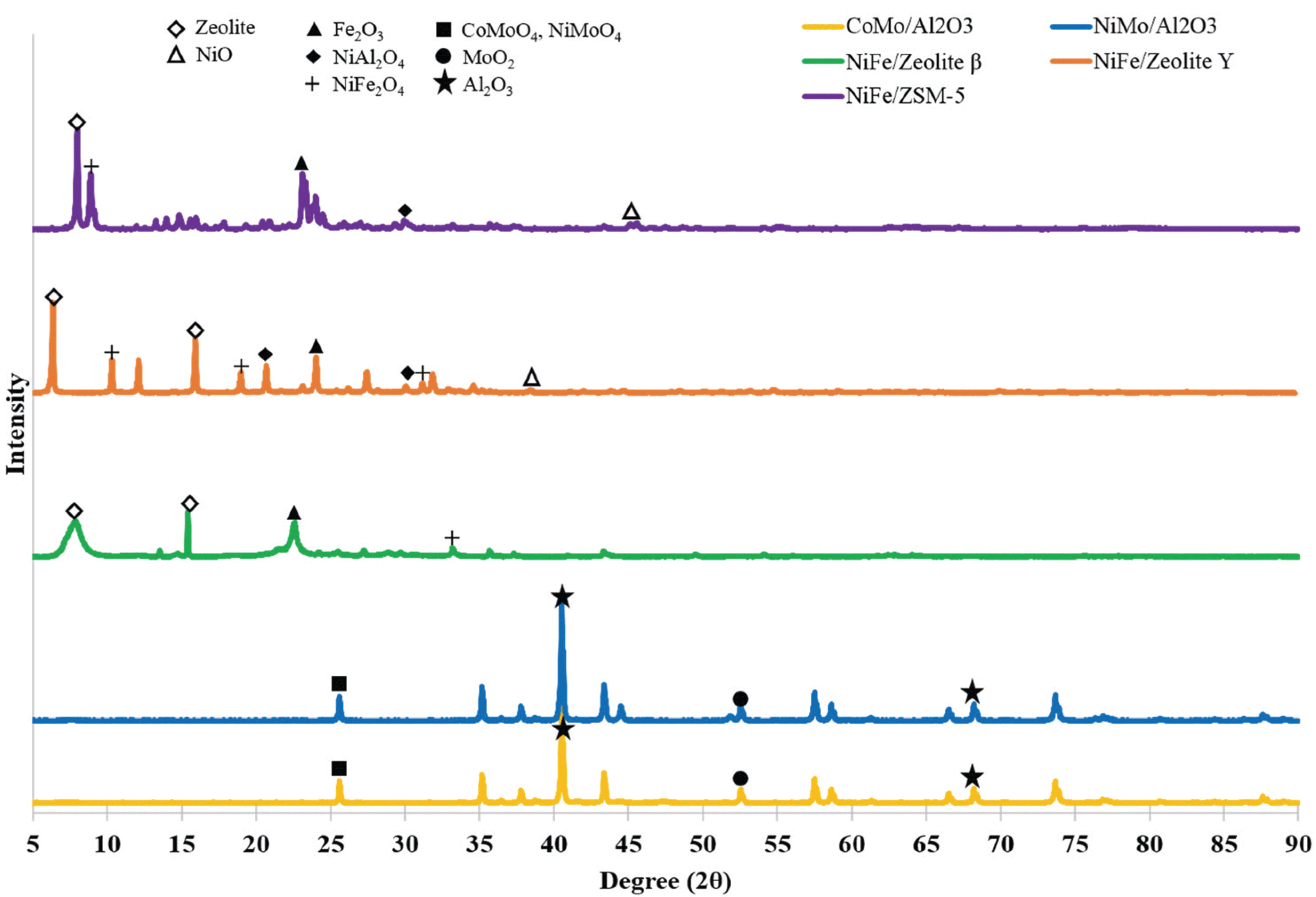
The impregnation of NiFe over the zeolite supports led to the formation of bifunctional catalysts (NiFe/ zeolite Y, NiFe/HBeta, and NiFe/ZSM-5) as well as led to the formation of metal oxide on the surface contained slight amount of crystalline aggregation as evidenced from the phase formation in the high angle XRD.
Fig. 4 presents the XRD peaks of CoMo/Al2O3, NiMo/Al2O3, NiFe/zeolite Y, NiFe/HBeta, and NiFe/ ZSM-5. CoMo/Al2O3 and NiMo/Al2O3 presented three typical metal oxide peaks at 2θ = 26° and 53°, with Al2O3 support peaks at 2θ = 41° and 68°. Compared to Al2O3 supports, zeolites peaks detected in lower angle, such as 2θ =6-8° and 16° (Zhang et al., 2012; Hunter et al., 2016). Moreover, differed zeolite resulted in different metal oxide forms; mainly consist of NiFe2O4 in NiFe/zeolite Y while NiAl2O4 and Fe2O3 in NiFe/ ZSM-5.
To test and compare the HDO activity of catalysts, HDO of waste wood bio-oil was conducted with those catalysts. The bio-oil was converted and produced heavy oil, light oil, char and gas. The yields of the HDO products obtained with different temperature and solvent concentration are shown in Tables 2-3 based on the weight of the bio-oil. The yield of each product revealed that the composition of the HDO products was considerably affected by the zeolite supports. Compared the data from three different supports (NiFe/HBeta, NiFe/zeolite Y and NiFe/ZSM-5), HBeta support showed thermally stable features according to the heavy oil yield from 300 to 400 °C (18.3-18.9 wt%). However, the char and gas strikingly increased with temperature, which meant both further decomposition by temperature and catalyst deactivation by coke deposition on surface are accelerated. Both NiFe/zeolite Y and NiFe/ZSM-5 showed the tendency of decrease heavy oil and light oil and strikingly increase in gas, while the char decrease in NiFe/ZSM-5 and increase in NiFe/zeolite Y. While the NiFe/HBeta, which has high specific surface area with high Si/Al ratio (Table 1), showed similar products yield with varied reaction conditions (Tables 2-3). According to the results, it is suggested that the catalyst high Si/Al ratio could inhibit the catalyst deactivation.
In case of commercial CoMo/Al2O3 and NiMo/Al2O3, the gas yield (12.5-21.6 wt%) is lower than that from zeolite supported bifunctional catalysts (17.3-42.5 wt%) with similar yield of heavy oil. It is suggested that these CoMo/Al2O3 and NiMo/Al2O3 catalysts deactivated less than NiFe/zeolite Y, NiFe/HBeta, and NiFe/ ZSM-5, which prevented that the further decomposition by the temperature.
As shown in Table 3, the heavy oil yielded less in lower concentration of ethanol. Since the waste wood bio-oil itself was too sticky (103 mm2/s, Tables 4-5), it produced huge amount of sticky tar-like char (14.6- 37.1 wt%). Also, these were affected by the particle size and specific surface area (Table 1). Small particle size with large specific surface area led frequent efficient collision, but in the batch type reactor, sticky feature of the bio-oil might not let the catalysts regenerated.
Typical fuel properties, such as acidity, heating value, and thermal stability, are improved in heavy oil after HDO via hydrocracking, hydrogenolysis, dehydration, hydrogenation and deoxygenation (Furimsky et al., 1986). Therefore, several improved physicochemical properties of heavy oil were measured and are displayed in Tables 4-5. The water content, which is major feature related to the heating value and combustion properties in the engine, is 19.3% in the waste wood bio-oil and decreased to 3.1-16.6 wt% in the heavy oils, via dehydration of the organic phase. Since the metal site of commercial catalysts, involved in dehydration well remained H2-reduced state, CoMo/ Al2O3 and NiMo/ Al2O3 worked better than prepared bifunctional catalysts (even though the zeolites are known as good at dehydration). The zeolites with high acidity (also concerned with dehydration reaction) also worked for reducing water content. But the zeolite framework structure tends to collapse under high temperature (Blakeman et al., 2014), the water content of heavy oil increased again in 400 °C. Moreover, the mesoporous alumina-silica support (e.g. MCM-41, SBA-15 etc.) presented higher dehydration than microporous catalysts (Shemfe et al., 2017). However, the zeolites used in this study had similar pore size and pore volume, the acidity (Si/Al ratio) would be highly affected to dehydration. While the solvent concentration presented not that notable effect on the variation of water content (Table 5).
The TAN of the heavy oil was significantly reduced (28-77 mg KOH/g oil) in comparison to that of waste woo bio-oil (150mg KOH/g oil). Despite acid removal, phenolic compounds should be measured as a weak acid by KOH. Therefore, the heavy oils still showed certain level of acidity. Viscosity is also the important factor when the fuel injected into an engine (Ochoa-Hernández et al., 2013). In this experiment, the viscosity of waste wood bio-oil decreased from 103 mm2/s to 43-86 mm2/s in heavy oils. This assumed that the catalysts could effectively convert the macromolecular compounds consisted of bio-oil into monomeric or low molecular compounds in the heavy oils.
The elemental compositions of the waste wood bio-oil and heavy oil were determined and are presented in Tables 4-5. Compared to the carbon (42.9 wt%), hydrogen (7.1 wt%), and oxygen (49.9 wt%) contents of the waste wood bio-oil, the carbon and hydrogen levels increased (57.0-68.7 wt%, and 7.5-10.0 wt%, respectively), while the oxygen level decreased (22.6-34.1 wt%) in heavy oils. Nitrogen and sulfur was not detected in almost of all the heavy oils, while some heavy oil contains less than 0.1 wt%. High carbon content and hydrogen content with low oxygen content resulted in high higher heating value (HHV). The HHV was calculated with the equation from previous study (Friedl et al., 2005) using dry basis C, H, and N contents. As a result, HHV of 13.7 MJ/kg (waste wood bio-oil) enhanced 22.6-31.2 MJ/kg in heavy oils.
The heavy oil obtained from 400 °C with CoMo/ Al2O3 presented the highest carbon content and the lowest oxygen level among those heavy oils, resulting in the highest HHV. However, despite the high carbon content in the heavy oils obtained from lower solvent concentration, the oxygen level also high due to the catalyst deactivation, resulted in lower HHV than the 25 wt% solvent mixture as a raw material.
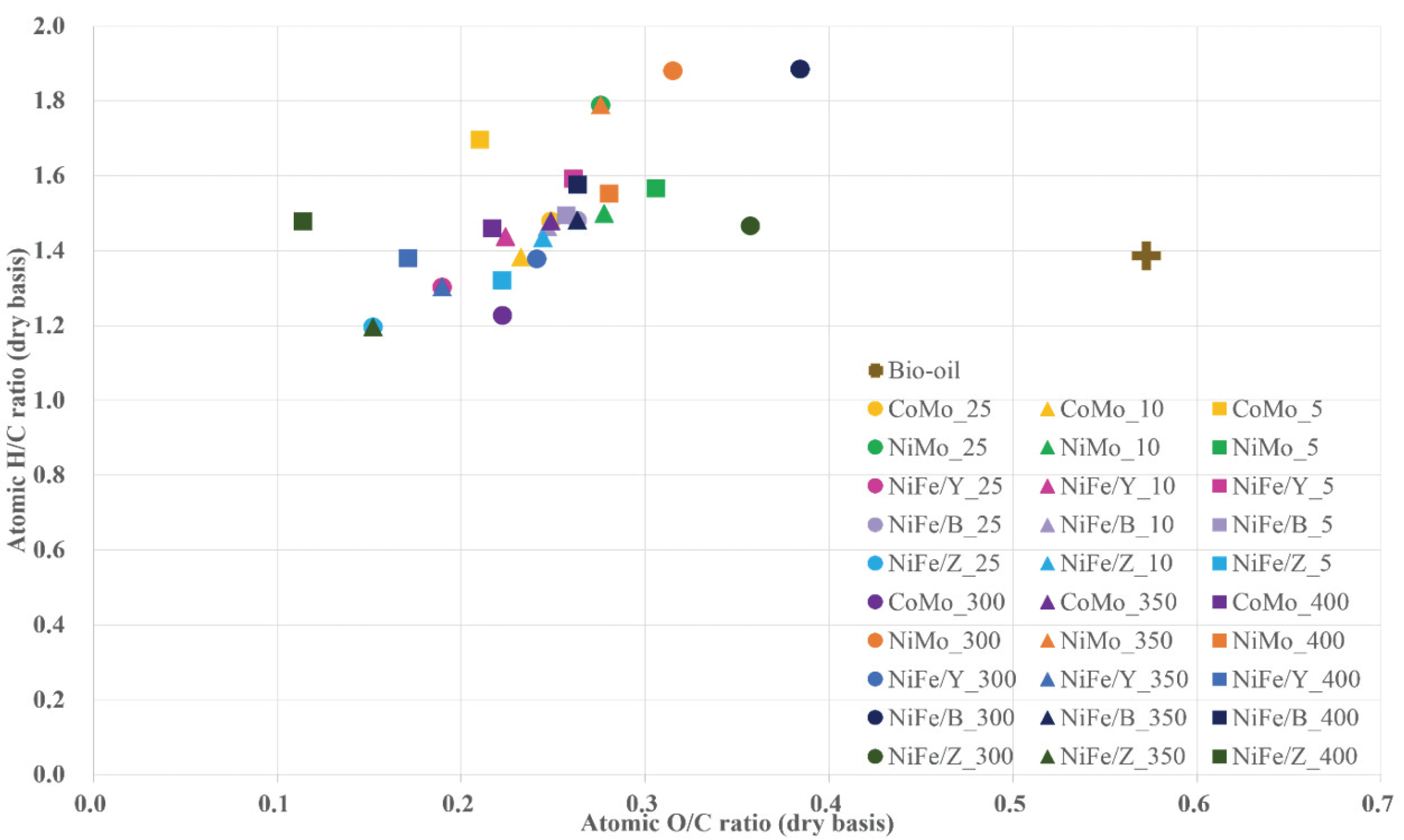
In Fig. 5, the Van Krevelen diagram, consisted of atomic O/C and H/C ratio to easily compare the deoxygenation and hydrogenation after the HDO reaction, of the bio-oil, and heavy oils was described. The atomic ratio of waste wood bio-oil (0.6) decreased ranged from 0.1-0.4. The lowest O/C ratio (0.1) was investigated in the heavy oil obtained from 400 °C with NiFe/ZSM-5. Zeolite supported bifunctional catalysts showed the tendency of decreasing the atomic O/C ratio with temperature, while CoMo/ Al2O3 and NiMo/ Al2O3 didn’t presented the trend with temperature. While some conditions reduce even the H/C ratio of waste wood bio-oil (1.4) to 1.2-1.4. NiFe/HBeta under 300 °C HDO presented H/C ratio of 1.9. When the bio-oil hydrodeoxygenated with this NiFe/HBeta, both hydrogenation and deoxygenation performed well (0.3-0.4 of O/C ratio and 1.5-1.9 of H/C ratio, respectively). Also NiMo/Al2O3 presented well hydrogenation (higher H/C ratio than that of waste wood bio-oil; 1.5-1.9).
As mentioned in previous section, high oxygen levels result in high acidity, viscosity, and low heating values, deoxygenation is the most important to the value-added utilization of bio-oil. During HDO process, deoxygenation, such as demethoxylation, dehydroxylation, or dehydration simultaneously occurred and led to decrease oxygen levels in bio-oil. The DOD was calculated based on the atomic O/C ratio. After the HDO, the O/C ratio of the bio-oil (0.6) decreased to 0.1-0.4 due to deoxygenation. There were no significant differences among the HDO temperature, except for using the NiFe/ZSM-5 catalyst. This catalyst has the smaller pore diameter, and when the temperature increased, some part of framework collapsed, but might retain its features to maintain metal active sites involved in deoxygenation. When the solvent concentration varied, NiFe/ zeolite Y and NiFe/HBeta showed opposite trend. It seemed to be due to coke deposition on the surface pore. High specific surface area of NiFe/HBeta was deactivated by coke and the NiFe/HBeta catalyst became aggregated. So it might not have change to collapse the zeolite framework (Chen et al., 1997). The trend is same in the water content, so it is clear that the acid sites of these catalysts related to the dehydration.
Typical compounds in bio-oil and heavy oils were identified from the GC/MS analysis and classified based on chemical structure and functional groups. Their relative amounts are presented in Table 6.
The generated compounds in heavy oil mainly consisted as phenolic compounds, such as phenol, cresol and guaiacol. The minor compounds, such as esters, ketones, and other phenolic compounds, were also present in heavy oils after the HDO. The heavy oils showed a 15.4-36.8% decrease in the total compounds compared to bio-oil. The decrease typically resulted from decarboxylation and further decomposition of acetic acid, vanillin, syringaldehyde and levoglucosan. According to previous study (Oh et al., 2017), acetic acid which was not converted into ethyl ester form, remained and separated in the light oil phase.
Generation of cresol and removal of alkyl-guaiacol suggest that catalysts can accelerate dealkylation or transalkylation. However, the remaining alkyl-guaiacol and alkyl-syringol as well as producion of 4-propylsyringol suggest that NiFe-based catalysts might not totally effective for reducing the activation energy of dealkylation, or decomposition. Moreover, abundant guaiacol and small amount of syringol remained in the heavy oil, despite demethoxylation to phenol. This result was due to the dealkylation, or demethoxylation via hydrogenation (Furimsky et al., 1986). The unstable and undesired oxygenated compounds in the bio-oil, such as acetic acid, vanillin and levoglucosan which could perform repolymerization during the reaction, were effectively converted into acetic acid ethyl esters, 2-methyl-2-cyclopenten-1-one, or 2(5H)-Furanone in the heavy oil via hydrogenation, decarbonylation, dehydroxylation, and ring opening, or low molecular gas phase. However, compared with previous study with mesoporous catalysts (Oh et al., 2017), the total amount of Furans were less in this results. It might be due to the different composition of bio-oil as well as the varied metal sites (from previously studied monometallic Ni (Oh et al., 2017) to Mo-based and bimetallic NiFe-based catalysts in this study).
4. CONCLUSION
In this study, the HDO of waste wood bio-oil using two commercial and three prepared bifunctional catalysts in batch type reactor were investigated. The porous zeolite supported NiFe-based catalysts yielded lower heavy oil than non-porous Mo-based catalysts. NiFe/ZSM-5, has the lowest specific surface area notably affected by temperature and solvent concentration. While, the fuel properties of heavy oil improved through HDO. Especially HHV of the heavy oil from CoMo/ Al2O3 under 400 °C, were even more improved 56.1 % than bio-oil. Considering with HHV and the heavy oil yield, the NiFe/zeolite Y, has higher specific surface area and low Si/Al ratio presented the most improved quality heavy oilat high temperature, while the solvent concentration slightly affected to the quality.