Original Article
Secondary Metabolites with Anti-complementary Activity from the Stem Barks of Juglans mandshurica Maxim
Zi-Jiang Li
2,3, Shilin Chen
2, Xiang-Hao Yang
2, Rui Wang
2, Hee-Jeong Min
4, Lei Wu
5, Chuan-Ling Si
2,4,5,†
, Young-Soo Bae
4,5,†
2Tianjin Key Laboratory of Pulp & Paper, College of Papermaking Science & Technology, Tianjin University of Science & Technology, Tianjin 300457, China
3Shandong Institute of Commerce and Technology, Jinan 250103, China
4Department of Forest Biomaterials Engineering, College of Forest & Environment Sciences, Kangwon National University, Chuncheon 24341, Korea
5Institute of Applied Chemistry, Jiangxi Academy of Sciences, Nanchang 330096, China
†Corresponding author: Young-Soo Bae (e-mail:
bae@kangwon.ac.kr, ORCID: 0000-0003-1108-9269)
†Corresponding author: Chuan-Ling Si (e-mail:
sichli@tust.edu.cn, ORCID: 0000-0003-1630-7800)
© The Korean Society of Wood Science & Technology.
Received: Nov 08, 2017; Accepted: Dec 22, 2017
Published Online: Mar 25, 2018
Abstract
Juglans mandshurica is a fast growing hard species, which is a tree in family of Juglandaceae and has a wide distribution in China, Korea and eastern Russia. Plant materials from J. mandshurica have extensively been used in folk medicines to prevent or cure gastric, esophageal, lung and cardiac cancer. As one chain of our searching for anticomplementary agents from natural sources, two epimeric ellagitannins, [2,3-O-4,4′,5,5′,6,6′,-hexahydroxydiphenoyl (HHDP))-(α,β)-D-glucose] (I) and pedunculagin (II) were purified from 70% acetone extracts of the stem barks of J. mandshurica by Thin Layer Chromatography and Sephadex LH-20 column chromatography approaches. The chemical structures of the isolated compounds were characterized by MS, NMR, and a careful comparation with published literatures. The epimeric ellagitannins I and II exhibited inhibitory properties against a classical pathway of complementary system with 50 % inhibitory concentrations (IC50) values of 65.3 and 47.7 μM, respectively, comparing with riliroside (IC50=104 μM) and rosmarinic acid (IC50=182 μM), which were used as positive controls. Thus, the work indicated both the two secondary metabolites possess excellent inhibitory activity and might be developed as potential anticomplementary chemicals.
Keywords: anticomplementary activity; epimeric ellagitannins; Juglans mandshurica Maxim; spectroscopic technique; secondary metabolites
1. INTRODUCTION
The human complement system plays an important role in the host defense system against foreign invasive organisms such as viruses, bacteria, and fungi, as well as an external wound. Its effects are normally beneficial to the host, but it can also cause adverse effects depending on the site, extent, and duration of complement activation (Cimanga et al., 1995). Activation of the system may lead to pathologic reactions in a variety of inflammatory and degenerative diseases such as multiple sclerosis, systemic lupus erythematosus, sjogren syndrome, dermatological disease, rheumatoid arthritis, and gout. Therefore, the modulation of complement activity is important and there is a need to develop anti-complementary agents from various sources such as plants (Park et al., 1999; Min et al., 2003).
Juglans mandshurica Maxim (Juglandaceae), a fast growing deciduous tree, is widely distributed in China, Siberia and Korean peninsula. The tree has been used as a folk medicinal plant for the treatment of esophageal, gastric, cardiac and lung cancer. It was reported that volatile constituents from the species inhibited the growth of the neighboring plants and can be developed for chemurgy (Min et al., 2003; Kim, 1994). Some of the valuable constituents such as α-tertalonyl glucopyranosides, naphthoquinones, naphthalenyl glycosides, flavonoids, galloyl glucopyranosides and diarylheptanoyl glucopyranosides have already been isolated from this species (Park et al., 2017; Wang et al., 2017; Min et al., 2003; Li et al., 2003). In addition, several studies have reported the anti-complement activity, inhibition of human immunodeficiency virus type 1 reverse transcriptase and ribonuclease H activities of J. mandshurica extract (Bi et al., 2016; Min et al., 2003; Min et al., 2000).
Thus, and as a part of a project aimed at discovering bioactive and structurally novel compounds from plant sources, the chemical constituents of J. mandshurica bark and their anti-complement activity were investigated.
2. MATERIALS and METHODS
2.1. Instruments
Melting points (uncorrected) were determined with an Electro Thermal 9100 apparatus. Optical rotations were measured on a JASCO DIP-1000 digital polarimeter in MeOH. IR spectra were obtained on a Perkin-Elmer BX FT-IR spectrometer in a KBr disk. UV spectra were recorded in MeOH (Jenway 6405 spectrophotometer). 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were recorded in MeOH-d4 with TMS (Tetramethylsilane) as an internal standard using a Bruker Avance DPX 400 spectrometer. MALDI- TOF-MS spectroscopy was measured on a Model Voyager-DE STR spectrometer.
Paper TLC analysis were carried out on DC-Plastikfolien Cellulose F (Merck Co.) plates and developed with t-BuOH-HOAc-H2O (3 : 1 : 1, v/v/v, solvent A) and HOAc-H2O (3 : 47, v/v, solvent B). TLC spots were detected by UV-light (254 and 365 nm) and by spraying with 1% FeCl3 (in EtOH) solution followed by heating.
2.2. Anti-complement Assay
Anti-complement properties of the isolated epimeric ellagitannins were evaluated by a method adopted from Yamada et al.(1985). A diluted solution of normal human serum (complement serum, 80 μL) was mixed with a gelatin veronal buffer (GVB2+, 80 μL) without or with samples. The mixture was pre-incubated at 37°C for 30 min, followed by adding sensitized erythrocyte (sheep red blood cells, 40 μL). After incubation under the same conditions, the mixture was centrifuged (4°C, 1500 rpm) and the absorbance of the supernatant (100 μL) was measured at 450 nm by a UV spectrometer (Libra S32, Biochrom). Each sample was dissolved in DMSO as negative control, while tiliroside and rosmarinic acid were used as positive controls. Anti- complement activity was determined as a mean of three independent trials and expressed as the 50% inhibitory concentration (IC50) values from complement- dependent hemolysis of the control (Jung et al., 1998; Oh et al., 2000).
2.3. Plant Material
The stem bark was stripped from a 10-year-old J. mandshurica tree grown in the experimental forest of Kangwon National University, Korea in April, 2005 and was identified by Prof. Wan-Keun Park, School of Forest, Kangwon National University, Korea. A voucher specimen has been deposited at the herbarium of Laboratory of Natural Products, Kangwon National University, Korea.
2.4. Extraction and Fractionation
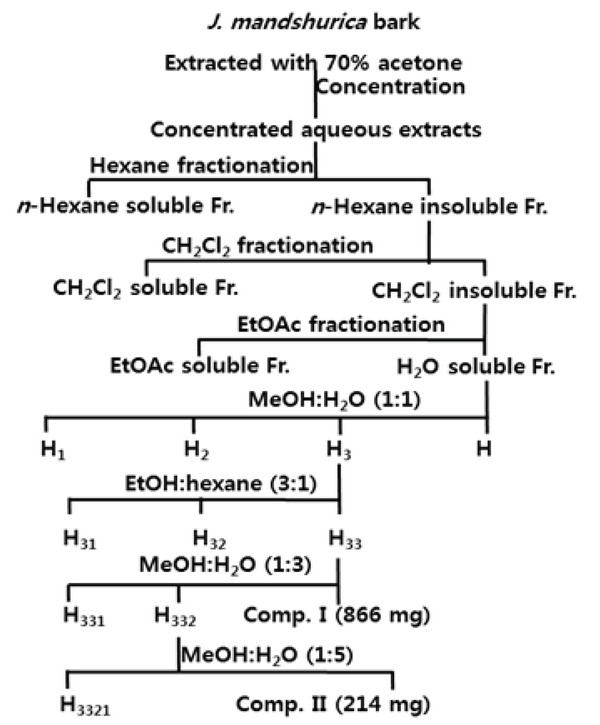
The bark of J. mandshurica was air-dried and ground with a Wiley mill. A precisely weighted amount (3.2 kg) was extracted in 70 % acetone aqueous solution (each 20 L for 72 h×3 times) at room temperature. The extracting solutions were filtered, combined, and concentrated with an evaporator under reduced pressure to afford the crude extract, which was suspended in water and then successively submitted to liquid–liquid fractions with in separation funnels using n-hexane, methylene chloride (CH2Cl2), ethylacetate (EtOAc) and n-buthanol (n-BuOH) (Si et al., 2017). For the detailed extraction and fractionation procedures see Fig. 1. The soluble fractions in n-hexane (15.6 g, yield 0.5%), CH2Cl2 (20.6 g, yield 0.6%), EtOAc (130 g, yield 4.1%) and H2O (337.6 g, yield 10.6%) were obtained after freezing-drying as powders.
Fig. 1.
Extraction, fractionation and isolation procedures of tannins from J. mandshurica stem bark.
Download Original Figure
2.5. Isolation of Ellagitannins
As shown in Fig. 1, a portion of the resulting H2O soluble Fr. (40.0 g) was applied to a Sephadex LH-20 column eluting with MeOH-H2O(1:1, v/v) to give four fractions: H1 (12.4 g), H2 (8.5 g), H3 (16.2 g) and H4 (2.8 g), which were sampled and their composition were monitored by Paper TLC. Fraction H3 was reapplied to a Sephadex LH-20 column using EtOH-hexane (3 : 1, v/v) as eluent for further purification to yield three subfractions and the third subfraction H33 (8.9 g) was stepwisely rechromatographed with MeOH-H2O (1 : 3, v/v) to give subfractions H331 (1.8 g) and H332 (6.2 g) and compound I (866 mg). Subfraction H332 was resubjected to a Sephadex LH-20 column and eluted with MeOH-H2O (1 : 5, v/v) for further separation to give subfraction H3321 (5.9 g) and compounds II (214 mg).
3. RESULTS and DISCUSSION
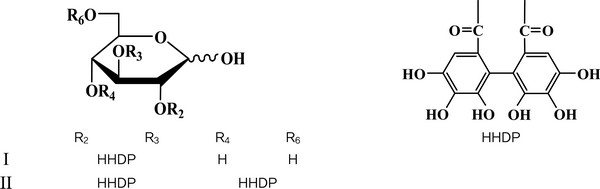
Compound I, which has never been reported from J. mandshurica previously, was isolated as a colorless amorphous powder with melting point of 112–114 °C and optical rotation of αD20 + 18.5° (c 0.1 in MeOH). On paper TLC, its Rf (Retention Factor) values are 0.68 (solvent A) and 0.77 (solvent B). Its molecular weight 482 and molecular formula C20H18O14 was determined based on the quasi-molecular ion peaks m/z [M+Na]+ at 505, [M+K]+ at 521, [2M+Na]+ at 987 and [2M+K]+ at 1003 in the MALDI-TOF-MS spectrum. The presence of phenolic hydroxyl group in the molecule was recognized from grey-green color with ethanolic FeCl3 solution on TLC(Imakura et al, 1985). In its IR spectrum, absorption bands at 3380, 1738, 1615, 1511, 1435, 1230, 1185, 875, 830, 735 (cm-1 in KBr) were observed. Its UV spectrum showed maxima at 262 nm (in MeOH). In 1H- and 13C-NMR spectra, A characteristic anomeric mixture of α- and β-D-glucose and connecting 4,4′,5,5′,6,6′,-hexahydroxydiphenoyl (HHDP) residues were observed. However, compound I, a pair of epimers, could not be completely separated by column chromatography though it appeared as only one spot on two-dimensional paper TLC. Its spectroscopic data were identical with those reported by Seikel et al, (1970), thus compound I was identified as 2,3-O-4, 4′,5,5′,6,6′-HHDP-(α/β)-D-glucose.
Compound II was obtained as a pale brown amorphous powder for the first time from this species. The melting point was 135–137°C. We found its optical rotation αD20 of +106° (c 0.1 in MeOH). Its IR and UV absorption bands were similar with those of compound I described above. Rf of this compound are 0.29 (solvent A) and 0.55 (solvent B). The MALDITOF- MS spectrum of compound II gave peaks of m/z [M+Na]+ at 807, [2M+K]+ at 823, [2M+Na]+ at 1591, [2M+K]+ at 1607, respectively, corresponding to the molecular formula C20H18O14. Similar to that of compound I, compound II was also an epimeric compound, which was existing in equilibrium as anomeic mixture of α- and β-D-glucoses as described in previously published literatures (Tsujita et al., 2017; Seikel et al., 1970). In the 1H-NMR spectrum of compound II, two sets of partially overlapped α- and β-D-glucose were confirmed. In addition, four sets of 4,4′,5,5′,6,6′,-hexahydroxydiphenoyl (HHDP) moieties were observed. The 13C-NMR of compound II also presented duplicated signals due to the presence of a pair of epimers. These 1H- and 13C-NMR data were coincided with those reported in literature (Seikel et al., 1970) and compound II was elucidated as pedunculagin consequently.
3.1. 2,3-O-4,4′,5,5′,6,6′-HHDP-(α/β)-D-Glucose (I)
Colorless amorphous powder; mp 112–114 °C; αD20 + 18.5° (c 0.1 in MeOH); IR (KBr) νmax cm-1 3380, 1738, 1615, 1511, 1435, 1230, 1185, 875, 830, 735; UV λ max (MeOH) nm 262; Rf : 0.68 (solvent A) and 0.77 (solvent B); MALDI-TOF-MS : m/z [M+Na]+ at 505, [M+K]+ at 521, [2M+Na]+ at 987 and [2M+K]+ at 1003; 1H- and 13C-NMR data were identical with those in the literature (Seikel et al., 1970).
3.2. Pedunculagin (II)
Pale brown amorphous powder; mp 135–137°C; αD20 + 106° (c 0.1 in MeOH); IR (KBr) νmax cm-1 3380, 1747, 1615, 1511, 1435, 1230, 1185, 875, 830, 735; UV λmax (MeOH) nm 260; Rf : 0.29 (solvent A) and 0.55 (solvent B); MALDI-TOF-MS : m/z [M+Na]+ 807, [2M+K]+ 823, [2M+Na]+ 1591, [2M+K]+ 1607; 1H- and 13C-NMR data were in agreement with those of the literature (Tanaka et al., 1993).
3.3. Anti-complementary Activity
Compounds I and II isolated from J. mandshurica stem bark were assayed for their anti-complement activity on the complement system of classical pathway (CP) in vitro and the results were summarized in Table 1. Both the two epimeric ellagitannins exhibited strong anti-complement activity with IC50 values were 65.3 and 47.7 μM, respectively, comparing tiliroside and rosmarinic acid 104 and 182 μM, respectively, which were used as positive controls. These facts suggested that the two epimeric ellagitannins could be used as anti-complement agents.
Table 1.
Anti-complementary effects of isolated epimeric ellagitannins by complementary system of classical pathway in vitro.
|
|
Sample |
IC50(μM)a |
|
Ellagi-tannins |
2,3-O-4,4′,5,5′,6,6′-HHDP-(α/β)-D-glucose (I) |
65.3 |
|
pedunculagin (II) |
47.7 |
|
Positive controls |
Tiliroside |
104 |
|
Rosmarinic acid |
182 |
Download Excel Table
4. CONCLUSION
By successive liquid-liquid fractionation and repeated TLC-monitored purification of 70% aqueous acetone extraction of the J. mandshurica barks, two ellagitannins were isolated and their chemical structures (Fig. 2) were elucidated as 2,3-O-4,4′,5,5′,6,6′-HHDP-(α/β)-D-glucose (I) and pedunculagin (II) based on their chemical and spectroscopic evidences, and a careful comparison with previously published data. Compounds I and II were epimeric ellagitannins and they were isolated as anomeric mixtures of α- and β-D-glucoses in the current work as described in literatures. It is noteworthy that this is the first report of compounds I and II from J. mandshurica.
The activity of the two epimeric ellagitannins (I and II) were evaluated by a classical pathway of complementary system assay, and our investigation results indicated that both the two compounds inhibited excellent effects and could be used as potential anti-complementary agents.
ACKNOWLEDGMENT
This work was kindly supported by National Key Research and Development Program of China (Grant No.2017YFB0307903), Foundation of Key Project of Research and Development Program of Jiangxi Province (No. 20171BBH80017 and 20171ACF60009), the Science and Technology Major Project Foundation of Jiangxi Academy of Sciences (2018-YZD1-05 and 2018-YZD2-18), Science Foundation for Young Doctors of Jiangxi Academy of Science (2016-YYB-07), and Introduction of Overseas Technical and Managerial Personnel Program of State Administration of Foreign Experts Affairs (20173600003), P.R. China.
References
Bi D.D, Zhao Y.C, Jiang R, Wang Y, Tian Y.X, Chen X.Y, Bai S.J, She G.M. Phytochemistry, bioactivity and potential impact on health of Juglans: the original plant of walnut. Natural Product Communications. 2016; 11(6):869-880.

Cimanga K, Bruyne T.D, Lasure A, Poel B.V, Pieters L, Berghe D.V, Vlietinck A, Kambu K, Tona L. In vitro anticomplementary activity of constituents from
Morinda morindoides. Journal of Natural Products. 1995; 8(3):372-378


Imakura Y, Kobayashi S, Mima A. Bitter phenyl propanoid glycosides from
Campsis chinensis. Phytochemistry. 1985; 24(1):139-146


Jung K.Y, Oh S.R, Park S.H, Lee I.S, Ahn K.S, Lee J.J, Lee H.K. Anti- complement activity of tiliroside from the flower buds of
Magnolia fargesii. Biological & Pharmaceutical Bulletin. 1998; 21(10):1077-1078


Kim T.W. The Woody Plants of Korea. 1994; Seoul, Korea: Kyohak Press. p. 58-59.

Li G, Xu M.L, Choi H.G, L S.H, Jahng Y.D, Lee C.S, Moon D.C, Woo M.H, Son J.K. Four new diarylheptanoids from the roots of
Juglans mandshurica. Chemical & Pharmaceutical Bulletin. 2003; 51(3):262-264


Min B.S, Nakamura N, Miyashiro H, Kim Y.H, Hattori M. Inhibition of human immunodeficiency virus type 1 reverse transcriptase and ribonuclease H activities by constituents of
Juglans mandshurica. Chemical & Pharmaceutical Bulletin. 2000; 48(2):194-200


Min B.S, Lee S.Y, Kim J.H, Lee J.K, Kim T.J, Kim D.H, Kim Y.H, Joung H, Lee H.K, Nakamura N, Miyashiro H, Hattori M. Anti- complement activity of constituents from the stem- bark of
Juglans mandshurica. Biological & Pharmaceutical Bulletin. 2003; 26(7):1042


Oh S.R, Kinjo J, Shii Y, Ikeda T, Ahn K.S, Kim J.H, Lee H.K. Effects of triterpenoids from
Pueraria lobata on immunohemolysis: β-D-glucuronic acid plays an active role in anticomplementary activity in vitro. Planta Medica. 2000; 66(6):506-510


Park S.H, Oh S.R, Jung K.Y, Lee I.S, Ahn K.S, Kim J.H, Kim Y.S, Lee J.J, Lee H.K. Acylated flavonol glycosides with anti-complement activity from Persicaria lapathifolia. Chemical & Pharmaceutical Bulletin. 1999; 47(10):1484-14686


Park S, Kim N, Yoo G.J, Kim S.N, Kwon H.J, Jung K.W, Oh D.C, Lee Y.H, Kim S.H. Phenolics and neolignans isolated from the fruits of Juglans mandshurica Maxim. and their effects on lipolysis in adipocytes. Phytochemistry. 2017; 137(8):87-93


Seikel M.K, Hillis W.E. Hydrolysable tannins of Eucalyptus delegatensis wood. Phytochemistry. 1970; 9(5):1115-1128


Si C.L, Gao Y, Wu L, Liu R, Wang G.H, Dai L, Li X.H, Hong Y.M. Isolation and characterization of triterpenoids from the stem barks of Pinus massoniana. Holzforschung. 2017; 71(9):697-703


Tanaka T, Tachibana H, Nonaka G, Nishioka I, Hsu F.L, Kohda H, Tanaka O. Tannins and related compounds. CXXII. New dimeric, trimeric and tetrameric ellagitannins, lambertianins A- D, from
Rubus lambertianus Seringe. Chemical & Pharmaceutical Bulletin. 1993; 41(7):1214-1220


Tsujita T, Matsuo Y, Saito Y, Tanaka T. Enzymatic oxidation of ellagitannin and a new ellagitannin metabolite from Camellia japonica leaves. Tetrahedron. 2017; 73(5):500-507


Wang T.M, Liu J, Yi T, Zhai Y.J, Zhang H, Chen H.B, Cai S.Q, Kang T.G, Zhao Z.Z. Multiconstituent identification in root, branch, and leaf extracts of Juglans mandshurica using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry. Journal of Separation Science. 2017; 40(17):3340-3452


Yamada H, Ohtani K, Kiyohara H, Cyong J.C, Otsuka Y, Ueno Y, Omura S. Purification and chemical properties of anti- complementary polysaccharide from the leaves of
Artemisia princeps. Planta Medica. 1985; 51(2):121-125